We spoke to you about it on 27 March in this same section: the Afssaps (French Health Products Safety Agency) has just published the Cosmetovigilance balance sheet for 2009. A report which was also studied by the FEBEA (Federation of the companies of the beauty), one of the representatives of the cosmetic companies, which transmitted us its analysis in the form of additional information, which we publish here in full.
March 31, 2010 What adverse reactions were reported to AFSSAPS in 2009? (FEBEA text)
First of all, it should be noted that in Cosmetovigilance 2009 results , l’ Afssaps announced the development of various documents: the accountability method, recommendations for the proper use of cosmetic products intended for consumers, the consumer warning campaign concerning skin whitening products and voluntary depigmentation, and the draft recommendations for the proper use of permanent hair dyes. It should also be noted that the information is more detailed since the 2007 balance sheet (published in 2008).
Number of adverse reactions received
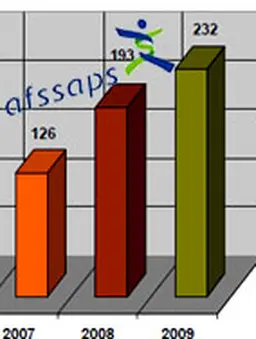
In 2009, 232 adverse reactions were reported to the AFSSAPS compared to 193 in 2008. This represents an increase of 20%. Between 2007 and 2008, this increase was 53% (126 versus 193). Over two years, the number of adverse reactions received has thus doubled.
Notifiers shall
Two new types of tax filers were identified this year: nurses and the DGCCRF (total 4%). The other declarants, already mentioned last year, are found in the same proportions EXCEPT industrialists who in 2008 and 2007 represented 1% of declarants and now represent 16% this could explain the increase in reported cases?
Gravity
The number of serious adverse …