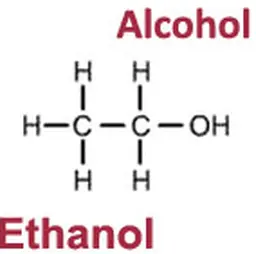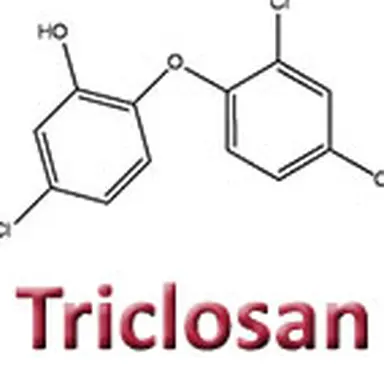
Triclosan (2,4,4’-trichloro-2’-hydroxy-diphenylether) is an antibacterial preservative that is also used as a deodorant agent. Long criticised for its harmful effects on health and the environment, it has just been – after several evaluations by European scientific experts – more strictly regulated and its use in cosmetic products has been limited.
Triclosan has a long history in cosmetics. Like many preservatives, it was first used widely in all kinds of products before arousing scientific suspicion and worry about its toxicity. Criticised more and more heavily, all around the world, it has been assessed several times by European experts, who decided to implement certain restrictive measures in order to better protect consumer safety. These measures have taken form in regulation 358/2014.
Suspicions aroused
Triclosan has long been known to persist in the environment. This bio-accumulative substance is accused of disrupting the development of flora and fauna, particularly in aquatic environments. Several studies have pointed to its endocrine-disrupting properties, which affect the natural regulation of oestrogenic and androgenic hormones and can have repercussions on human fertility. We also know that it is a potential skin irritant, and some cases of contact allergies have been reported. It is also reputed to be a photosensitiser.
Generating resistance
The main charge against triclosan, however, has to do with its formidable efficacy. As a broad-spectrum antimicrobial and bactericidal agent (it acts on a large number of germs), it has been targeted by several research teams due to its ability to trigger bacterial resistance (as is the case …