Following the mandate given by the European Commission to the SCCS in February 2020, in the framework of the evaluation of ingredients listed as endocrine disruptors, the Scientific Committee has just published its preliminary Opinion on the safety of Octocrylene (UV filter). It has been adopted by written procedure on 15 January 2021 and is open for comments until 15 March 2021.
Background
Following the publication of a list of 14 substances suspected of acting as endocrine disruptors to be assessed on a priority basis, and the subsequent call for data, the European Commission asked the SCCS for its Opinion on the safety of five substances: Resorcinol, Homosalate, Propylparaben, Benzophenone-3, and Octocrylene.
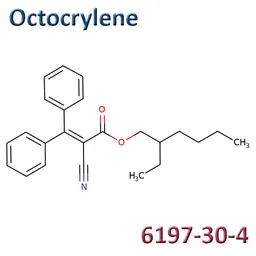
In cosmetic products, Octocrylene (CAS No.6197-30-4, EC No.228-250-8) is currently regulated as a UV-filter in sunscreen products in a concentration up to 10% (as acid) (Annex VI/10).
For an exhaustive background information, see the article
• Endocrine disruptors: 5 requests for SCCS Opinions, CosmeticOBS, 10 February 2020
Related Ingredients
Opinion
1. In light of the data provided and taking under consideration the concerns related to potential endocrine disrupting properties of Octocrylene, does the SCCS consider Octocrylene safe when used as a UV-filter in cosmetic products up to a maximum concentration of 10% (as acid)?
On the basis of safety assessment, and considering the concerns related to potential endocrine disrupting properties of Octocrylene (CAS No.6197-30-4), the SCCS considers that it is safe at concentrations of up to 10% when used individually or together as a UV-filter in cosmetic products, i.e. in sunscreen cream/lotion, sunscreen pump spray, face cream, hand cream …