
Nano-UV filters
Nanomaterials used in cosmetics must meet very specific requirements. Article 13 of the Cosmetics Regulations requires their presence in a product to be reported when it is notified on the CPNP portal. Article 16 also requests products containing nanomaterials to be notified six months before they are placed on the market. Finally, Article 19 requires their presence to be indicated in the INCI list on the product packaging by the word [nano] after the name of the ingredient concerned. All this presupposes being able to correctly identify and characterise a nanomaterial, which in many respects is still a difficult task with uncertain results. The principle being that a nanomaterial can only be used if it is listed in one of the annexes of the Cosmetics Regulation, after having been deemed safe by the Scientific Committee for Consumer Safety (SCCS), the European Commission has drawn up a Catalogue of nanomaterials, on the basis of notifications from industry under Article 16. A sort of roadmap for the safety assessment of these substances, before they are added, or not, in an annex to the Regulation, divided into three parts: colorant, UV filters and nanos with other functions. This dossier, updated in real time as soon as new information becomes available, brings together the detailed sheets for nano-ingredients that appear in version 2 of the Catalogue of nanomaterials, with the regulations that currently apply to them, their possible classification in the CLP Regulation, the history and progress of their assessment…
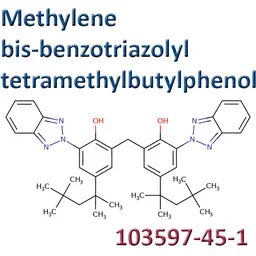
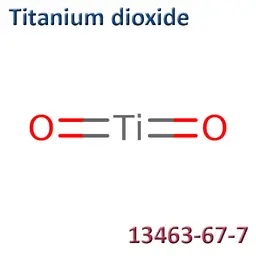
![Titanium dioxide [nano]](https://d2aabgjce9enf.cloudfront.net/main/media/components/7/b/7b95a1f545f420c9e41743173515381ad66dc911--sm-noborder.webp)

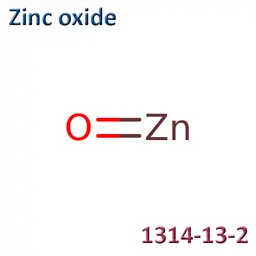
![Zinc oxide [nano]](https://d2aabgjce9enf.cloudfront.net/main/media/components/b/a/ba2378686bcef9431d5aae7854747827ba8b83e6--sm-noborder.webp)