When a substance is classified as CMR, it is a priori prohibited for use in cosmetic products, unless an exceptional exemption is granted after evaluation by the SCCS (Article 15 of the Cosmetics Regulation 1223/2009). Between the classification proposal and its entry into force, the industry has time to adapt: reformulate the products involved, adapt its supply chain, organise the withdrawal of non-compliant products. This timetable, detailed by Amanda Isom of Bloom Regulatory at the 5th Cosmetics Regulation and Compliance Congress organised by ERPA, provides the markers for acting at the right time.
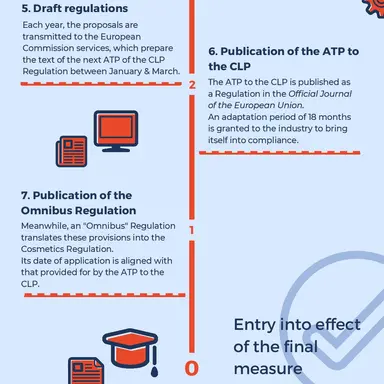
1. Entry in the Register of Intentions
Following concerns expressed most often by a Member State or a hazard identified under the Biocides or Plant Protection Products Regulations, ECHA enters the substance in the Register of Intentions.
• Time period before the final measure comes into effect: approximately 5 years.
2. Submission of the dossier
From 6 to 12 months after inclusion in the Register of Intentions, the entity that notified ECHA of the request submits a dossier justifying it.This dossier is subject to a conformity check by ECHA within 6 months.
3. Public consultation
All interested parties are invited to submit comments on the classification proposal or any relevant information on the substance, usually within 60 days.
4. Evaluation of ECHA’s Risk Assessment Committee (RAC)
All available data are forwarded to the RAC, which evaluates them before issuing its Opinion, within 4 to 12 months (maximum allowed: 18 months). This Opinion usually prefigures the action that will ultimately be taken.
It is at this point that the industry may decide to defend a substance, by presenting a safety dossier which will be evaluated by the SCCS.
• Time period before the final measure comes into effect: approximately 3 years. …