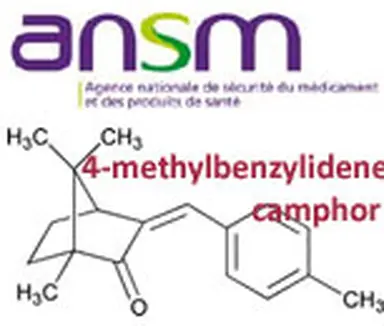
Is using 4-Methylbenzylidene Camphor, a UV filter currently allowed up to 4% in cosmetic products, but also listed as an endocrine disruptor substance, safe? Yes, for the SCCS European experts. No, for the French ANSM, which questions the reasoning and the safety margins stated by this committee.
The evaluation of the risk linked to using 4-Methylbenzylidene Camphor in cosmetic products comes after a referral of the ANSM (previously the Afssaps) by the Ministry for Health in 2009 on the risk due to harmful for reproduction and/or endocrine disruptors substances in cosmetic products.
Several opinions by the European Scientific Committee for the Safety of Consumers (SCCS) on 4-Methylbenzylidene Camphor (4-MBC) have been released between 1996 and 2008. The result is that this committee of experts, which is a major factor in the new regulations of cosmetic products, thinks that using 4-MBC up to a content of 4 % in cosmetic products can be considered as without any risk to the human health.
Challenged methods and figures
In its report, released on beginning May 2012, the Agency says it has had a critical view on all the SCCS opinions, as well as on the available studies linked to 4-methylbenzylidene camphor (4-MBC). It challenges the SCCS conclusions on several points:
• The guessed cutaneous levels of absorption,
• The displayed no observed adverse effect level (NOAEL): there is no justification for its figure, and it does not seem relevant, as per the ANSM,
• The safety margin (42.5),
•The choice …













