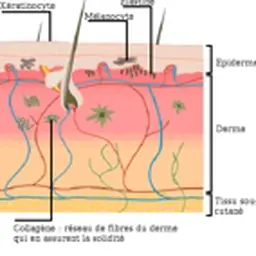
Widely used in many industries and present in many consumer products, nanomaterials, nevertheless, are a concern, especially due to their possible effects on consumers’ health. Many questions, not yet so many answers: however, for their application in cosmetics, their regulatory statute is currently being rewritten, and their definition becomes more precise.
As per the European Regulation CE No 1223/2009 (which replacee completely the former Directive since July 2013), nanomaterial means “an insoluble or biopersistant and intentionally manufactured material with one or more external dimensions, or an internal structure, on the scale from 1 to 100 nm.”
Nevertheless, this Regulation states that this definition should be adapted if a uniform definition for nanomaterials at the international level is agreed upon.
As a first step of this process, the European Commission has endorsed a proposal, on 18 October 2011, that follows the words by Janez Potočnik, a member of the Commission responsible of the environment: a “universal definition of nanomaterials that shall be used for all regulatory purposes.”
This proposal describes nanomaterials as “a natural, incidental or manufactured material containing particles, in an unbound state or as an aggregate or as an agglomerate and where, for 50% or more of the particles in the number size distribution, one or more external dimensions is in the size range one nm - 100 nm.”
This definition will be reviewed, in the light of experience and of scientific and technological developments.
Nevertheless, the European Regulation has new requirements for nanomaterials based on this definition:
• Cosmetic products containing nanomaterials shall be notified to the Commission six months prior to being placed on the market (including their identification and specification, and their toxicological profile)
• All ingredients present in the form of nanomaterials shall be clearly indicated in the list of ingredients. Since 2013, the names of such ingredients shall be followed by the word “nano” in brackets.
For further information
• The full text of the European Regulation 1223/2009
• The full text of the Commission Recommendation on the definition of nanomaterial
Also see
• The “Nanomaterials: back from dusk to plain light” article