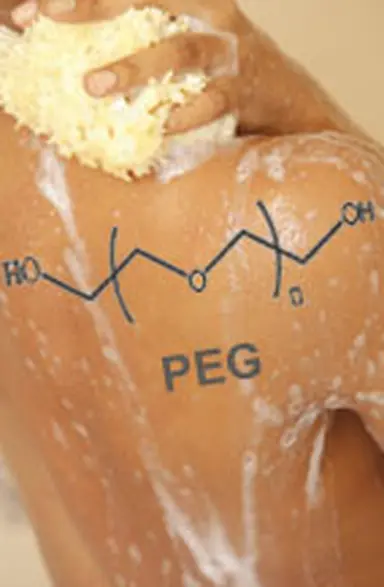
Acronym for PolyEthylene Glycol. By extension, "PEGs" refers to all the cosmetic ingredients derived from it, that can be recognized by the letters "PEG" in their official name.
PEGs
can
be
synthetic
or
claimed as being from
natural origin
; nevertheless, the "natural" is quite far, due to their manufacturing process, which includes ethoxylation.
This chemical reaction
involves
a
grafting
of
Ethylene
Oxide molecules
on
the
basic raw material
at
very
high
temperatures
and under
high
pressure
.
Why they are used
The derivatives of Polyethylene glycol are widely used in
cosmetic products
, mainly as
humectants
to "retain" water and prevent drying, in the product itself as on the skin.
Their name, formed from their acronym followed by their molecular weight, gives a clue as per their origin and aspect. A molecular weight below 500: it is a liquid. Above 500: oil or wax. PEG-8 is thus a liquid
humectant
.
Their esters are used as
emulsifiers
or
surfactants
(which allow for an homogenous mixture of non miscible raw materials as oil and
water
). Therefore, they can be found in almost any kind of cosmetics supplied as an
emulsion
, from shower gel to care creams, shaving creams or toothpastes.
Why they are criticized
According to most scientists and health authorities, these compounds do not in themselves involve a real health hazard, although they may possibly serve as a support to other chemicals, some carcinogenic, mainly present as impurities at vey low doses.
Most are fairly well tolerated by skin, but some have an
irritating
potential if used in large quantities. Making skin more permeable, some have also the property to increase the absorption by skin of other ingredients found in cosmetics.
They are mainly criticized because of their manufacturing process, one of the most polluting processes for the environment used in the cosmetic industry, and proof is established they are not easily biodegradable.
Easy to find them in a list of ingredients: look for "PEG" in capital letters.













