![Par Ben Mills (Travail personnel) [Public domain], via Wikimedia Commons](https://d2aabgjce9enf.cloudfront.net/main/media/content/5/b/5b62847bc4229696df593a6b14899855ab56385e--md-noborder.webp)
Boric acid (boric acid or orthoboric acid) is a weak acid often used as an antiseptic, insecticide, neutron absorber in nuclear power plants to control the fission rate of uranium, and as a precursor to other chemical compounds. It takes its name from one of its components, boron. Its gross formula is as follows: H3BO3 or B(OH)3. When it is in the form of ore, it is referred to as sassolite. It exists as colourless crystals or as a white powder dissolving in water.
Boric acid is produced mainly from borate ore by its reaction with sulphuric acid. The largest source of borates in the world is an open-pit mine located in Boron, California, USA. Boric acid was first produced by Wilhelm Homberg (1652-1715) from borax, under the action of mineral acids, and was given the name'sal sedativum Hombergi'. Free acid is present in native form in some volcanic areas such as Tuscany, the Lipari Islands and in Nevada, its effluents are mixed with steam from cracks in the earth's crust. Boric acid is also a constituent of many minerals (borax, boracite, boronatrocalcite and colemanite).
Uses : - Medical and biological uses. It can be used as an antiseptic for burns or cuts and is sometimes used in ointments and ointments, or is used in a very dilute solution as an eye bath (boric acid water). As an antibacterial compound, boric acid has been prescribed as a treatment for acne. - Boric acid can be used to treat yeasts and fungal infections such as candidiasis (vaginal fungal infections) by filling eggs with boric acid powder that will be inserted into the vaginal cavity at bedtime for three to four consecutive nights. It has also been used in prevention of athlete's foot, by inserting powder into socks or stockings, and in solution, it may be prescribed to treat certain forms of otitis externa (ear infection) in humans or animals. The preservative in urine bottles (red cap) in the UK is boric acid. Sodium borate, a mild antiseptic, combined with other suitable components, can also be proposed for external use for eye diseases, such as dry eyes. - Lithium borate is the lithium salt of boric acid used in the laboratory as a buffer for the gel commonly used in nucleic acid electrophoresis buffers (called TAE and TBE buffers). It can be used for DNA and RNA electrophoresis, polyacrylamide gel and agarose gel. It is also often used as a relatively low toxic insecticide, for the extermination of cockroaches, termites, ants, fleas and many other insects. It can be used directly as a powder for fleas and cockroaches, or mixed with sugar or jelly for ants. It is also a component of many commercial insecticides. In this use, particularly in the case of cockroaches, boric acid powder is applied in areas frequented by insects. Fine particles cling to the paws of insects and subsequently cause fatal chemical burns. Boric acid is marketed for this use in residential areas in urban areas infested by cockroaches. - Use in the nuclear industry. Boron has a high neutron absorption capacity, but with the disadvantage, above a certain threshold, of increasing the risk of radiolysis of water. - Other jobs. Borates and boric acid have been used since the ancient Greek period for cleaning, food preservation and other activities. In the traditional jewellery and welding (plumbing) industry, boric acid has been or is still used in combination with denatured alcohol to reduce surface oxidation and the importance of oxidation on metals during metallurgy and welding operations. Silicone sealant was originally manufactured by adding boric acid to silicone oil. Boric acid is commonly used by amateur pyrotechnicians - in solution in alcohol - to give the flame a light green colour. It is also used in India and around the world to control dust on sports fields, to reduce friction and to increase the speed of play.
On this basis, in particular, on 16 February 2007, boric acid and its salts were classified as'Reprotoxic category 2' in the Annex to the 30th TPA of Directive 67/54822,23. As a result, its use has decreased significantly.
In cosmetic products or more generally beauty products
Boric acid has been used as a pH adjusting agent in the form of buffer solutions, for example Gifford solutions.
Examples of formula
Acid solution - Boric acid : 12.4 - Potassium Chloride: 7.4 - Demineralized water: Qsp 1000
Basic solution - Sodium carbonate: 21.2 - Demineralized water: Qsp 1000
Uses of Gifford solutions
| Acid solution | Basic solution | pH obtained |
| 30cc | 0cc | 5 |
| 30cc | 0.05cc | 6 |
| 30cc | 0.1cc | 6,2 |
| 30cc | 0.6cc | 7 |
| 30cc | 1cc | 7,2 |
| 30cc | 1.5cc | 7,6 |
| 30cc | 2cc | 7,8 |
| 30cc | 2.4cc | 8,4 |
The solutions proposed by Palitzch and Sorenson are based on the same principle.
But the most frequent uses were mainly related to its bacteriostatic properties. It has been used as an antimicrobial preservative in many preparations for many years now. However, its low bactericidal power has limited its use as a preservative to the benefit of other substances such as parabens. However, its bacteriostatic properties have made its use rather frequent. It is found in children's products such as diaper rash lotions. Its use is mentioned in deodorant products, toothpastes or nail bleaching products. But the most common use is in eye make-up removers or face lotions. As regards the lotions contour of the eye, one finds its use as regulator of the osmotic pressure of the products.
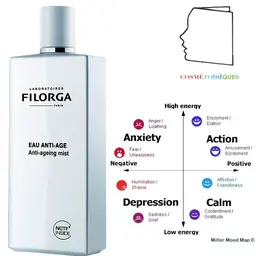
One of the best known uses is in Water Precious Spending©.
|
Contribution made by Jean Claude Le Joliff A biologist by training, Jean Claude Le Joliff has been an R&D man for many years. Successively in charge of R&D, then Research and Innovation in a large French cosmetics and luxury group, and after an experience of creating a research centre (CERIES), he turned to innovation management. He was also Associate Professor at the University of Versailles Saint Quentin (UVSQ) and remains a lecturer in several specialized courses: ISIPCA, IPIL, ITECH, UBS, UCO, SFC etc. He is the founder of inn2c, an R&D and Innovation consulting company. Consultant for several international companies, he has actively participated in projects such as Filorga, Aïny, Fareva, and many others. He created the Cosmétothèque®, the industry's first conservatory of crafts and know-how. |