In this second part, we will discuss the evidence of efficacy of this ingredient, the formulation elements, as well as a review of the state of the art, i.e. a fairly complete inventory of the different types of CACs available and their effects. We will also deal with a rather particular series, lipophilic esters based on PCA, such as , a kind of physiological esters because they are biomimetic.
Some Evidence of CADP Effectiveness
This molecule has been the subject of numerous studies concerning its efficacy. This chapter summarizes only a few of them.
There are numerous demonstrations of the hydrating effect of PCA. In this table (figure 5), you will find an example of evaluation of these properties by Corneometry, one of the reference techniques to evaluate this effect, even if it is not the most suitable. Indeed, the electrolytic character of the molecule does not predispose it very well to this mode of evaluation.
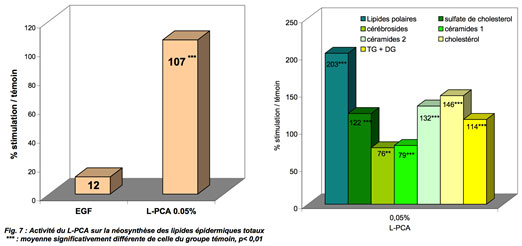
Beyond this effect, other techniques can complement these observations. For example, let's look at the effect of PCA on epidermal lipids.
Principle: lipid neosynthesis is evaluated by radioactive counting of radiolabelled acetate incorporated into the various epidermal lipids of whole human skin discs in culture. Under the experimental conditions of these tests, there is a strong increase in the synthesis of total epidermal lipids.
In another study, particular attention was paid to comparing the activities of L-PCA with gluconic acid on filaggrin maturation. The effect of the test products on filaggrin maturation is evaluated by Western Blot analysis (specific labeling of mature filaggrins (38kDa) with anti-filaggrin monoclonal antibody and chemiluminescence revelation). The test was performed on reconstructed epidermis placed in a specific culture medium and cultured at 37°C. After 7 hours of incubation, the culture medium is removed and replaced with a fresh medium containing or not the two test products in two concentrations.
The BCP in Formulation
Beyond its use as a moisturizing agent in skin care cosmetics, certain properties of this molecule must be taken into consideration in the formulation process. PCA salts, molecules resulting from the association of a trace element with pyrrolidone carboxylic acid, are distinguished by their electrolytic character, inducing a specific behaviour in formulation. Therefore, precautions should be taken in formulation. PCA salts have a preference for slightly acidic media and will be more stable in a pH range of 4.0 to 7.0. Apart from these values, they dissociate to form a precipitate.
If its electrolytic properties make it an interesting substance in specific applications, such as the stabilization of emulsions based on silicone surfactants, conversely, these same properties will make it a delicate substance to formulate in the presence of form systems susceptible to the behaviour of electrolytes. The problems are primarily issues of compatibility with the gelling agents used in formulation. Within the PCA salts themselves, there are variations in formulation behaviour related to their degree of covalence. It will therefore be necessary to differentiate between monovalent and divalent PCA salts.
The preferred gelling agents are vegetable and biotechnological gums, compatible with both monovalent and divalent PCAs. In this family, we find xanthan gum, gellan, carrageenates, sclero-tium, cellulose and its derivatives, sodium alginate, starch. The use of synthetic polymeric gelling agents such as carbomers and certain acrylates should be the subject of particular attention.
In emulsions, the electrolytic behavior of PCA salts will lead to the same problem as conventionally, variable with anionic and cationic H/E type emulsions, better with nonionic emulsions. In W/O emulsions, they will have a rather beneficial effect, even being able to replace advantageously all or part of the salts commonly used as magnesium sulphate or sodium chloride. The interest in siliphyl systems has already been mentioned previously.
Like most electrolytes, PCA salts may interact with certain molecules contained in plant extracts. These are molecules with a high molecular weight, such as tannins, proteins, mucilages and certain other plant polysaccharides. These incompatibilities lead to color variation or precipitate formation.
The PCA in all its forms
Expert in the field of PCA, UCIB - Solabia Group offers a wide range of molecules (trace elements, amino acids, fatty alcohols) associated with this physiological vector. These numerous PCA derivatives have different and complementary actions. Here are a few examples.
1. PCA and trace elements
- PCA OF SODIUM : Nalidone®. Action: physio-hydrating reference. - PCA POTASSIUM : > > > > > > > > > > Kalidone®. Actio: physio-regulating and hydrating. > > > > > > > > > > Aquaregul K®. Action:"Triple action" moisturizer, osmoregulator of intra and extracellular water flow. - PCA OF MAGNESIUM : > > > > > > > > > > Magnolidone®. Action: physio-energizing, special anti-fatigue skin. > > > > > > > > > > Mg-Relax®. Action: Yin-Yang anti-stress complex, relaxing and regenerating mixed care, prevention of the appearance of the first visible signs of aging. - PCA OF CALCIUM : Calcidone®. Action: physio-restructuring, special for attacked skins. - ZINC PCA : Zincidone®. Action: physio-seborator of oily skin. - COPPER PCA : Cuivridone®. Action: physio-seborator of oily skin, to be coupled to zinc PCA. - PCA OF MANGANESE : Mangalidone®. Action: mitochondrial physioprotector, optimization of the skin's natural defenses. - PCA OF ALUMIMIUM : Aludone®. Action: physio-astringent, special for sensitive skin. - PCA ASSOCIATION : Physiogenyl®. Action: physiological oligo-mineral catalyst, real breakfast of the skin.
2. PCA and amino acids
- ARGININE PCA: > > > > > > > > > > Argidone® 50. Action: = physio-regenerating, activator of metabolic reactions. > > > > > > > > > > Argisens®. Action: biological amplifier of sensory pleasure. - PCA OF LYSINE: Lysidone®. Action: physio-regenerating, strengthening the protection of cutaneous cellular metabolism.
3. PCAs and alcohols: see below Physio-Ester®.
4. Physio-complexes
- BUTYLENE GLYCOL (AND) OCTYLDODECYL PCA (AND) MENTHYL PCA : Cryogenyl ®. Action: Refreshing and restructuring physical-active.
Esters of pidolic acid, Physio-Ester®
Finally, let us mention lipid derivatives of PCA which constitute true physio esters, because biomimetics on the basis of the PCA molecule. These are fatty esters obtained by esterification of pidolic acid with fatty alcohols leading to original molecules.
Process for obtaining
Physio-Esters® are obtained by esterification of L-PCA with a fatty alcohol having a straight or branched carbon chain length between C12 and C22.
They are manufactured using a patented microwave synthesis process. This synthesis, qualified as soft, to oppose it to that obtained by organic solvents (toluene…), calls upon the specific properties of microwaves which, by optimising the reactivity of substrates after heating (by a supply of energy at the molecular level), favour the elimination of the water vapour formed.
Physio-Esters® are composed entirely of saturated fatty chains and are very stable to oxidation. In addition, they have excellent skin and eye tolerance. There are several varieties in this range of lipophilic derivatives of PCA.
Physio-Esters® are mainly characterized by their non-greasy touch and their spreading properties. They make it possible to formulate both emulsified and anhydrous preparations with particular touches. Moreover, the bi-lipidic structure of these derivatives gives them biomimicry activities on the restructuring of the skin barrier. This is how Ceramidone® stimulates the synthesis of total lipids, particularly ceramides 2.
These derivatives have quite specific properties on a case-by-case basis. This is for example the case of Laurydone® for its moisturizing effect.
Or MYRISTIDONE® for its effect on oily skin problems.
Or WAXIDONE®, a remarkable substitute for vegetable waxes and oils to improve touch. The optimized combination of the lauric and behenic ester of L-PCA also increases the breaking point of lipsticks and compact foundations while maintaining a melting texture.
Finally, let us mention an original and exclusive association integrating the PCA. Myristyl PCA (obtained by esterification of L-pyrolidone carboxylic acid and myristic alcohol), δ-viniferin and other polyphenols resulting from a biotechnological bioconversion process from an extract of Japanese knotweed (Polygonum cuspidatum). SANISKIN® offers a multiparametric approach by targeting four of the five essential pillars to combat oily skin: modulating sebaceous gland activity, protecting sebum quality against oxidation, limiting the proliferation of Propioniacnes and reducing skin inflammation.
Since its discovery in 1882, pyrrolidone carboxylic acid has therefore undergone a prestigious evolution, as shown by the more than 10,000 publications dedicated to PCA and its derivatives to date. To date, it is still a central element in the formulation of moisturizing specialties, and it is quite likely that current evaluation methods will allow us to discover new properties for this remarkable ingredient.
|
This contribution is possible thanks to the free collaboration of the Solabia Group .
|

 Founded in 1972, the Solabia Group designs and manufactures molecules and active ingredients for the cosmetic, pharmaceutical and nutraceutical industries, peptones and protein hydrolysates for bio-industries and microbiological diagnostic reagents for food, cosmetic, pharmaceutical and environmental laboratories. The Solabia Group has a coherent set of complementary skills such as biotechnology, fine chemistry, plant extraction and microbiology. The cosmetics department of the Solabia group, trend decoder, has built integrated solutions based on a well differentiated and convergent scientific expertise (biotechnology, fine chemistry and extraction), by developing active ingredients and concept-formulas. Among other things, PCA Science (PCA, salts and esters) developed by the UCIB entity of the Solabia group.
We would particularly like to thank Jean-François Molina who helped us to make this contribution. Jean-François is currently Sales & Marketing Director within the Solabia group. He obtained his engineering degree in biotechnology at the University of Aix-Marseille and his Masters in Product Innovation Marketing in 1989. For nearly 25 years within the Solabia group, he has successively held the position of Export Manager, Marketing Manager and now supervises the overall sales and marketing activities of the Solabia group's subsidiaries in the USA, Germany and Brazil.
Founded in 1972, the Solabia Group designs and manufactures molecules and active ingredients for the cosmetic, pharmaceutical and nutraceutical industries, peptones and protein hydrolysates for bio-industries and microbiological diagnostic reagents for food, cosmetic, pharmaceutical and environmental laboratories. The Solabia Group has a coherent set of complementary skills such as biotechnology, fine chemistry, plant extraction and microbiology. The cosmetics department of the Solabia group, trend decoder, has built integrated solutions based on a well differentiated and convergent scientific expertise (biotechnology, fine chemistry and extraction), by developing active ingredients and concept-formulas. Among other things, PCA Science (PCA, salts and esters) developed by the UCIB entity of the Solabia group.
We would particularly like to thank Jean-François Molina who helped us to make this contribution. Jean-François is currently Sales & Marketing Director within the Solabia group. He obtained his engineering degree in biotechnology at the University of Aix-Marseille and his Masters in Product Innovation Marketing in 1989. For nearly 25 years within the Solabia group, he has successively held the position of Export Manager, Marketing Manager and now supervises the overall sales and marketing activities of the Solabia group's subsidiaries in the USA, Germany and Brazil.