ECHA has just launched a public consultation to collect comments on its proposal to include seven SVHCs (Substances of Very High Concern) in the REACH authorisation list. To date, four can still be used in cosmetic products: D4, D5 and D6, and Trimellitic Acid. Comments can be sent until 5 June 2020.
ECHA is considering recommending four substances that can be used in cosmetics products for the European Commission to include in the Authorisation List (Annex XIV to REACH).
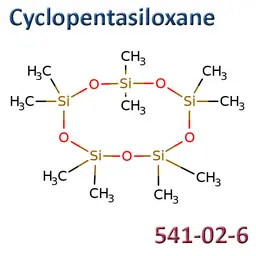
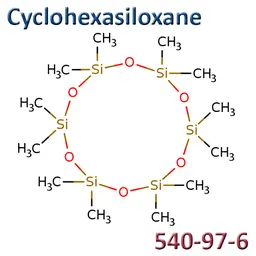
Octamethylcyclotetrasiloxane (D4), Decamethylcyclopentasiloxane (D5), Dodecamethylcyclohexasiloxane (D6)
D4 (INCI: Cyclotetrasiloxane / CAS: 556-67-2 / EC: 209-136-7), D5 (INCI: Cyclopentasiloxane / CAS: 541-02-6 / EC: 208-764-9) and D6 (INCI: Cyclohexasiloxane / CAS: 540-97-6 / EC: 208-762-8) were included in the candidate list for authorisation in June 2018.
As a reminder
• D4 has been prohibited for use in cosmetic products since the publication of the first Omnibus Regulation on CMRs (n°2019/831).
• D5 is part of the list drawn up by the European Commission of substances suspected of having endocrine disrupting properties to be evaluated on a priority basis by the SCCS, with a level 2 priority.
• D4 and D5 are already listed (by Regulation 2018/35) in Annex XVII of the REACH Regulation, which limits them to 0.1% in cosmetic rinse-off products since January 31, 2020.
• D4, D5 and D6 are furthermore subject to an identical proposed restriction (no more than 0.1%) in leave-on products.
Related Ingredients
Benzene-1,2,4-tricarboxylic acid 1,2-anhydride (trimellitic anhydride; TMA)
TMA (INCI: Trimellitic Anhydride / CAS: 552-30-7 / EC: -) …