
Labelling “new” allergens as Europe will require is one thing, but getting this new labelling accepted in every country in the world is quite another. And while in some regions the problem seems to have been solved in advance, in others it may prove to be much more complex, and a source of a few “red tape” problems. At CEAC 2023, Maxime Jacques, from Cosmetics Europe, mapped out the different situations that can be expected. Practical information sheet.
The day when cosmetic ingredients (and their labelling) will be regulated in exactly the same way throughout the world is still more of a dream than a near reality. And this will certainly add to the difficulties when the European Allergens Regulation comes into force.
This is what Maxime Jacques has illustrated with four maps, drawn up on the basis of an assessment of the situation carried out by Cosmetics Europe.
Ingredient regulations around the world
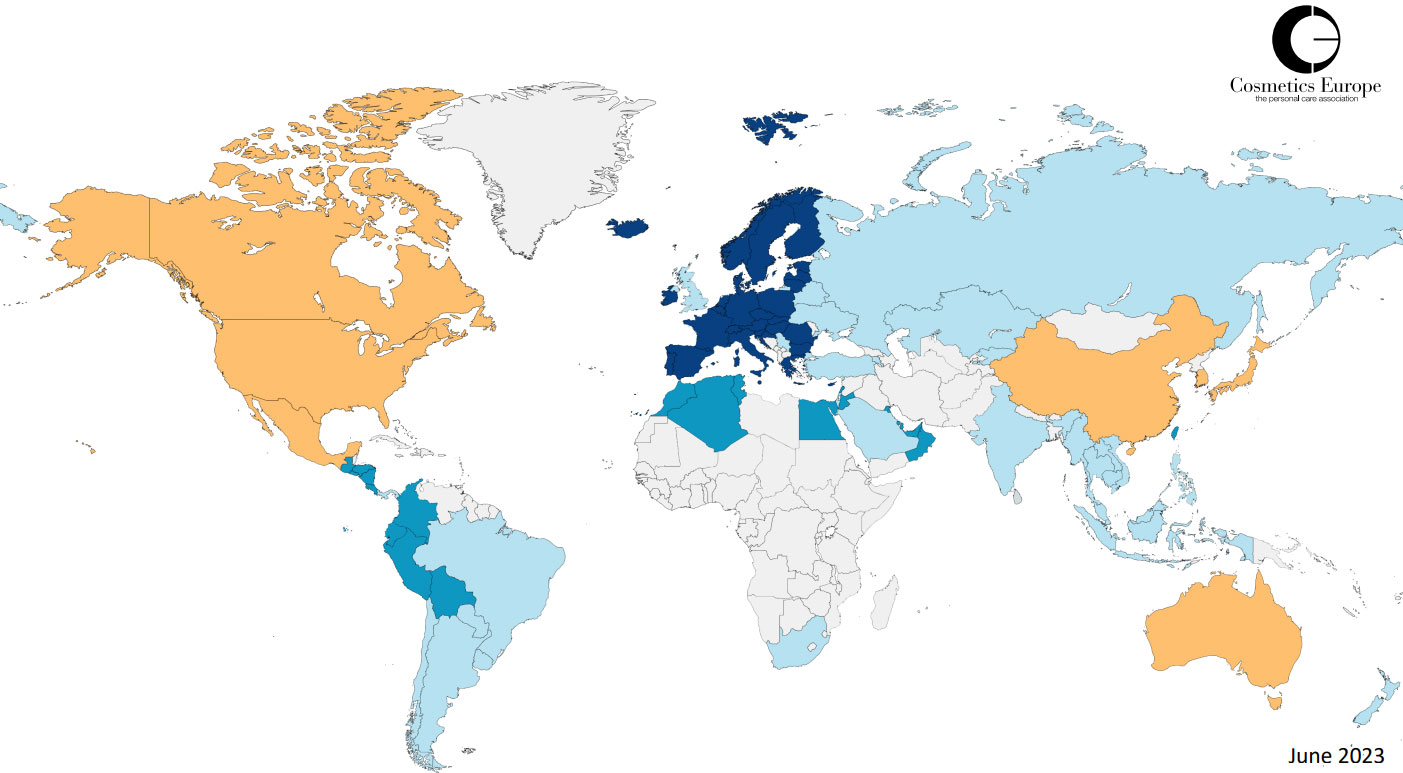
• In dark blue, the European Union (EU).
• In azure blue, the countries which have a direct reference to the annexes of the European Cosmetics Regulation: when they are updated, the modifications are automatically implemented.
• In light blue, the countries that progressively reflect EU regulations. They have the same management of ingredients, but most of the time adapt to the latest European developments with a certain delay.
• In orange, the countries which have a local framework and where the management of ingredients is different from that of the EU. But this does not mean that there is no compatibility. For example, in Canada, the system is not the same, but ultimately most of the EU requirements, with …














