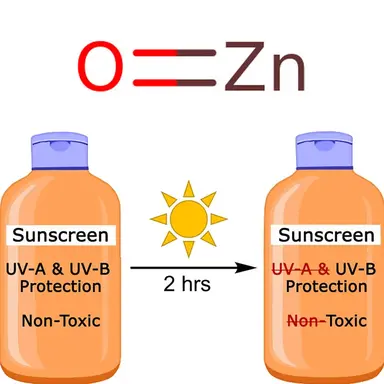
According to a recent study published in Photochemical & Photobiological Sciences, the Official Journal of the European Photochemistry Association and the European Society for Photobiology, after a two-hour exposure to UV light, zinc oxide, whether in nano or non-nano form, causes significant photodegradation of the small molecules of sunscreen filters, leading to higher levels of toxicity.
Abstract of the study
Sunscreen safety and efficacy is generally evaluated based upon the properties of the individual chemicals in a formulation. However, the photostability of sunscreens has been shown to be highly dependent on the mixture of chemicals present.
To better understand how sunscreen formulation influences stability, and to establish a foundation for probing the influence of zinc oxide additives, the research team formulated five different small-molecule based ultraviolet-filter (UV-filter) mixtures with a Sun Protection Factor (SPF) of 15.
These mixtures contained active ingredients approved in either the United States or European Union and were designed to represent formulations of actual products on the market.
The researchers evaluated the photostability and toxicity of these mixtures in the absence and presence of zinc oxide after UV exposure for two hours.
Changes in UV absorbance were minimal for all five small-molecule-based mixtures without zinc oxide. The presence of either micro- or nano-sized zinc oxide caused significant small-molecule photodegradation and the degraded mixtures exhibited higher levels of toxicity in embryonic zebrafish assays.
This study suggests that caution must be taken when formulating sunscreens containing both zinc oxide and small-molecule UV-filters to avoid unintended consequences during use.
The full study, published on 14 …













