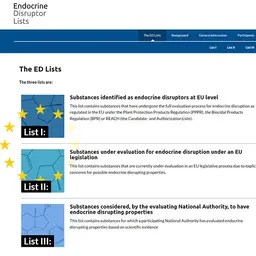
The European Union has just transmitted to WTO a draft regulation to ban the use of three fragrance allergens in cosmetic products. The text should enter into force mid-2017, but a time period from 2 to 4 years will be granted to the industry to adjust its products.
Notification date
5 January 2017
Content
This draft Commission Regulation would ban three fragrance allergens: HICC, Atranol and Chloroatranol which were found to be unsafe for use in cosmetic products by the Scientific Committee on Consumer Safety, and it is aimed to amend Annex II to Cosmetics Regulation 1223/2009.
The text of the future regulation
Whereas
1. The Scientific Committee on Consumer Safety (SCCS) concluded in its Opinion of 26-27 June 2012 that 3-and 4-(4-Hydroxy-4-methylpentyl) cyclohex-3-ene-1-carbaldehyde (HICC), with the INCI name of Hydroxyisohexyl 3-Cyclohexene Carboxaldehyde, 2,6-Dihydroxy-4-methyl-benzaldehyde (atranol) and 3-Chloro-2,6-Dyhydroxy-4-methyl-benzaldehyde (chloroatranol) should not be used in cosmetic products as they are the fragrance allergens which caused the highest number of contact allergies cases in past years.
2. Consequently, there is a potential risk to human health and those substances should therefore be prohibited in cosmetic products.
3. It is appropriate to provide for reasonable periods of time in order for the industry to adapt to the new prohibitions and for this reason no longer place on the market and withdraw from the market products concerned which contain one or more of the prohibited substances. When determining those periods of time, due account should also be taken of the potential risk of those products to human health.
4. In particular, the exceptionally complex and lengthy procedure for fragrance reformulation and consumers’ concerns over the change of the olfactory properties of fragrances should be reflected in longer than usual duration of the deadline given to the industry for adjustments of products. Manifestations of contact allergies to fragrances are normally confined to skin. Consumers with contact allergy to fragrance allergens often are aware that they cannot tolerate scented products on their skin and therefore can avoid them.
5. Annex II to Regulation (EC) No 1223/2009 should therefore be amended accordingly.
6. The measures provided for in this regulation are in accordance with the opinion of the Standing Committee on Cosmetic Products.
Articles
Article 1
Annex II to Regulation (EC) No 1223/2009 is amended in accordance with the Annex to this regulation.
Article 2
From [date = 2 years after the date of entry into force] cosmetic products containing one or more of the substances prohibited by this Regulation shall not be placed on the Union market.
From [date = 4 years after the date of entry into force] cosmetic products containing one or more of the substances prohibited by this Regulation shall be withdrawn from the Union market.
Article 3
This Regulation shall enter into force on the twentieth day following that of its publication in the Official Journal of the European Union.
This Regulation shall be binding in its entirety and directly applicable in all Member States.
Proposed modification of Annex II to Cosmetics Regulation
In Annex II to Regulation (EC) No 1223/2009, in the table, the following three entries are added:
|
|
Substance identification |
||
|
Reference number |
Chemical name/INN |
CAS number |
EC number |
|
1380 |
3-and 4-(4-Hydroxy-4-methylpentyl) cyclohex-3-ene-1-carbaldehyde (HICC)* |
51414-25-6/
|
257-187-9/
|
|
1381 |
2,6-Dihydroxy-4-methyl-benzaldehyde (atranol)** |
526-37-4 |
|
|
1382 |
3-Chloro-2,6-Dyhydroxy-4-methyl-benzaldehyde (chloroatranol)*** |
57074-21-2 |
|
|
*From [date = 2 years after the date of entry into force]. From [date = 4 years after the date of entry into force] all cosmetic products containing that substance shall be withdrawn from the Union market.
|
|||
Proposed date of adoption
Mid-March 2017
Proposed date of entry into force
20 days from publication in the Official Journal of the EU
From 2 years after the date of entry into force cosmetic products containing one or more of the substances prohibited by this Regulation shall not be placed on the Union market.
From 4 years after the date of entry into force cosmetic products containing one or more of the substances prohibited by this Regulation shall be withdrawn from the Union market.
Final date for comments
60 days from notification
For further information
• See the
draft regulation amending Annex II to regulation (EC) No 1223/2009 of the European Parliament and of the Council on cosmetic products – Part I
• See the
draft regulation amending Annex II to regulation (EC) No 1223/2009 of the European Parliament and of the Council on cosmetic products – Part II