A beautiful tanned complexion is still associated with good health and prosperity, at least in our Western countries. However, thanks also to our way of life that keeps us indoors, winter is synonymous with pale complexion, and summer, or exposure under the tropical sun, is synonymous with dangers due to repeated exposure to the UV radiation from sun. Is self-tanning the answer? Its flagship molecule is dihydroxyacetone (DHA, as it is known). Is this "magic bullet molecule" risk-free?
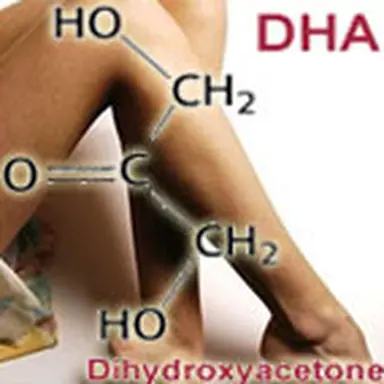
People interested in chemistry may understand that Dihydroxyacetone comes as acetone in which two hydrogen atoms have been replaced (substituted) by two hydroxyle groups; it is then a cetotriose. It is generally maunfactured by bioconversion of Glycerin , taken from corn, sugar cane, wheat, rape, beet or palm oil … This is why a "natural origin" is often referred to on labels for DHA, as it is à la mode.
Dihydroxyacetone is by far the main active ingredient in self-tanning products (often along with Erythrulose for more aesthetic results). In a cosmetics galenic form of gel or lotion, it allows for the getting, within 4 to 6 hours, of a "tanned effect", more or less deep, depending on its concentration in the product: after application, skin is gradually coloured until it seems to have a natural tan, without any exposure to sunrays.
It is also used in spray cans, especially in beauty salons, for a more uniform application and a more even aspect.
How does it do?
DHA reacts with the cutaneous keratine, or precisely with the aminoacids of the skin's proteins, by a Maillard reaction. This reaction produces brown pigments, called melanoidines, which give the skin its brown complexion. …













