The ANSM has just published a decision banning several Apy Skin Solutions brand products: the National Agency for the Safety of Medicines and Health Products states that they contain ingredients prohibited by cosmetic regulations, irritants and allergenics, and that they can cause serious adverse reactions.
The decision is dated February 25, 2019 and was released on March 7. It suspends the manufacture, export, wholesale distribution, placing on the market free of charge or against payment, holding for sale or distribution free of charge, advertising of several Apy Skin Solutions products, and the withdrawal of products.
The products concerned
• TCA peeling 10%
• TCA peeling 12.5%
• TCA peeling 15%
• TCA peeling 20%
• TCA peeling 25%
• Arbutine & Mequinol
• Acide kojique & Mequinol
• Mequinol cristal
• Jessner Peel
• Jessner Peel modified
The reasons for this decision
• The products TCA peeling 10%, TCA peeling 12.5%, TCA peeling 15%, TCA peeling 20%, TCA peeling 25% contain trichloroacetic acid, prohibited in cosmetics.
• The products Arbutine & Mequinol, Kojic acid & Mequinol, Mequinol crystal, with a lightening purpose, contain 2 to 10% mequinol, whereas this substance is only authorised in artificial nail preparations at a concentration of 0.02%.
• The products Jessner Peel and Jessner Peel modified contain salicylic acid and resorcinol in concentrations much higher than those permitted by cosmetic regulations.
• Mequinol and resorcinol are also irritating and allergenic substances that may pose a risk to users.
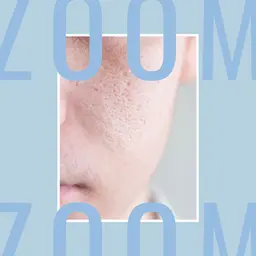
• An adverse reaction resulting from the use of TCA peeling 25% consisting of second-degree facial burning with post-inflammatory hyperpigmentation has been reported to the ANSM.
• Apy Skin Solutions has not notified its products to the European Commission and has not fulfilled its obligations to ensure consumer safety.
For further information
• See the full text of the ANSM Health Police Decision.