
You may have them in your cupboards. Maybe, you use them, still. Beware: they are dangerous, illegal, harmful… and are the subject of a recall or ban by the European sanitary authorities. Underneath, you may find all the cosmetic products targeted by the RAPEX alert system this week: an antifungal lotion containing a prohibited ingredient.
Anti-fungal lotion
Brand: Evterpa 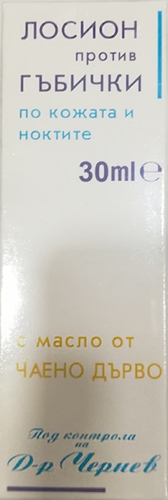
Name: Lotion with tea tree oil for fungal skin and nails
Batch number: EV01072019
Barcode: 3800201864916
• Country of origin: Bulgaria
• Notifying country: Bulgaria
Risk type: chemical
According to the ingredients list and product information file, the product contains boric acid (declared value: 0.05%), which releases boron.
Ingestion or contact with an excessive quantity of boron may harm the health by damaging the reproductive system or the unborn child.
The product does not comply with the Cosmetic Products Regulation.
Measures taken by economic operators: destruction of the product, withdrawal of the product from the market (by manufacturer)
Source
• Rapid Alert System - Weekly Reports, Report 12, European Commission, March 27, 2020













