On 11 September 2024, the European Chemicals Agency (ECHA) published the Netherlands’ intention of an harmonised classification and labelling (CLH) for Hydroxycitronellal (fragrance ingredient), notably as Repr. 1B.
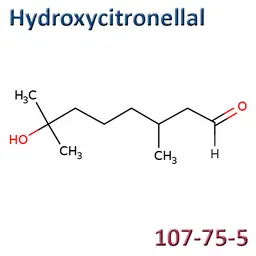
7-hydroxycitronellal (CAS No.107-75-5, EC No.203-518-7, INCI: Hydroxycitronellal) is an ingredient listed in the Cosing, with the function Perfuming.
It is listed in Annex III/72 of European Cosmetics Regulation 1223/2009 which limits its maximum concentration in products (other than oral products) to 1.0% and requires it to be declared in the INCI ingredients listwhen its concentration exceeds 0.001% in leave-on products and 0.01% in rinse-off products.
It currently has no entry in Annex VI of the European CLP Regulation 1272/2008.
The Netherlands intends to propose to classify the substance as follows:
• Skin Sens. 1B, H317 - May cause an allergic skin reaction
• Repr. 1B, H360D - May damage the unborn child
If the process is completed and this classification becomes effective, this substance could be listed as reprotoxic and therefore as a CMR, and its use could be banned in cosmetic products in Europe, unless the ban is waived following an opinion from the SCCS.
Related ingredient sheet
Sources
• ECHA Weekly - New intentions and proposals to harmonise classification and labelling, ECHA, 11 September 2024
• Registry of CLH intentions until outcome - 7-hydroxycitronellal, ECHA, 10 September 2024