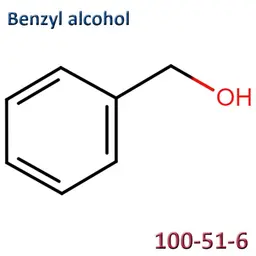
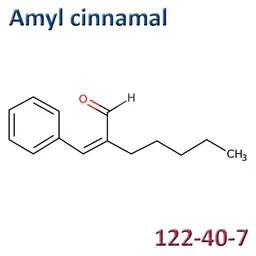
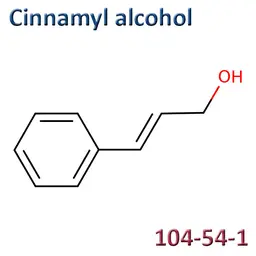
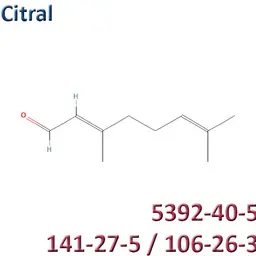
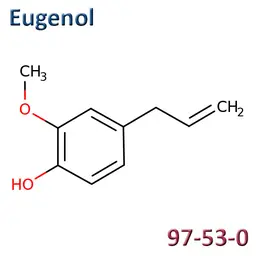
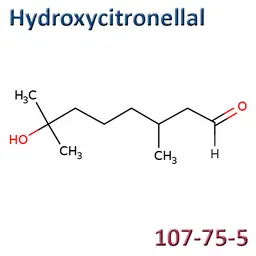
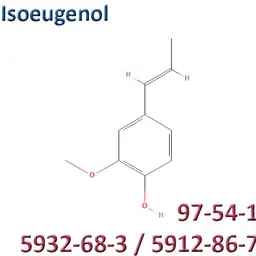
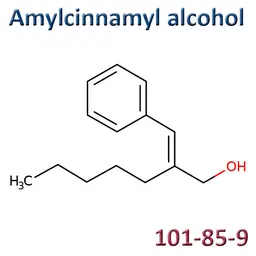
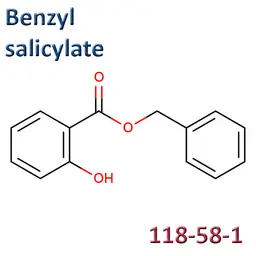
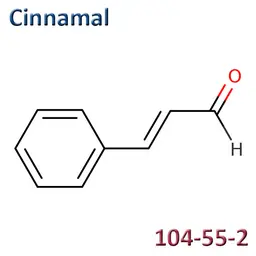
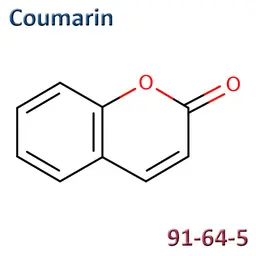
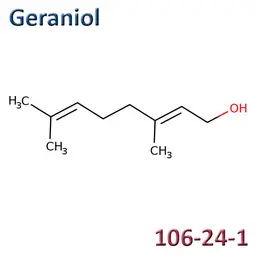
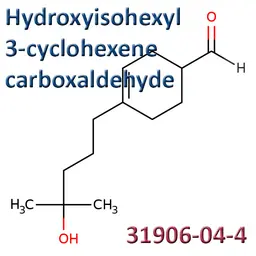
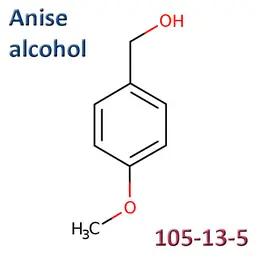
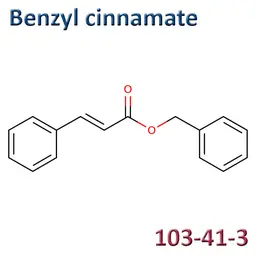
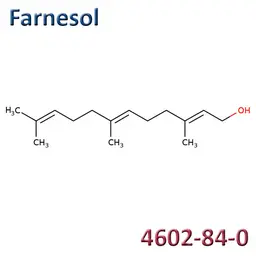
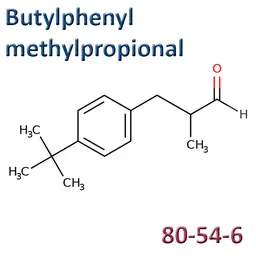
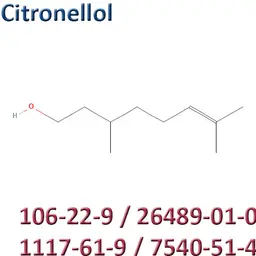
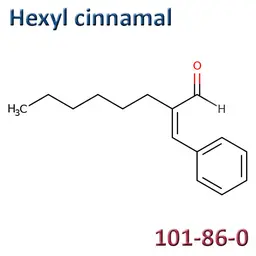
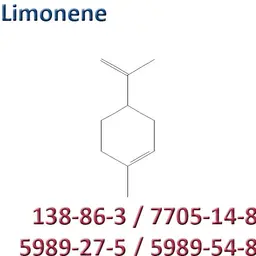
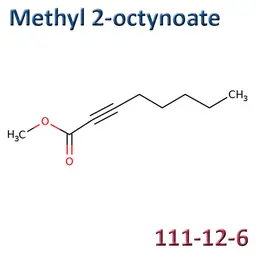
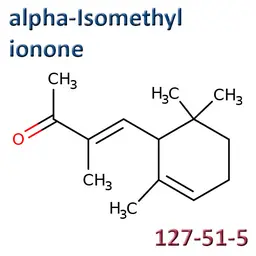
The labelable allergens

They are among the cosmetic ingredients most often denounced by the press or consumer organisations for their potential to harm health. They are omnipresent in products of all categories, especially as soon as they contain a perfume. Article 19 of the Cosmetics Regulation 1223/2009 provides, in point (g)(ii), that “Perfume and aromatic compositions and their raw materials shall be referred to by the terms ‘Parfum’ or ‘Aroma’” in the list of ingredients that must appear on the labelling of a cosmetic product, but “the presence of substances, the mention of which is required under the column ‘Other’ in Annex III, shall be indicated in the list of ingredients in addition to the terms ‘Parfum’ or ‘Aroma’.” The text thus designates the “labelable allergens” which must therefore appear in the list of ingredients as soon as they are present at more than 0.01% in rinse-off products and at more than 0.001% in leave-on products. This dossier contains detailed data sheets for all the substances involved, with the regulations applicable to them, IFRA restrictions, regulatory developments to be expected… A list that is subject to change, in line with changes in regulations, and which this dossier keeps up to date in real time.