Silicon is a chemical element of the crystallogen family, Si symbol and atomic number 14. It is the most abundant element in the earth's crust after oxygen, 25.7% of its mass, but it is comparatively present in relatively small quantities in the matter that makes up living organisms. On the other hand, it is present in cosmetic products.
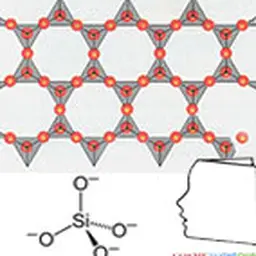
It does not exist in nature as a pure body, but as compounds: silicon dioxide (SiO₂), silica (in sand, quartz, cristobalite, etc.) or other silicates (in feldspar, kaolinite, etc.).
Its name derives from Latin flint, silicis which means rock or flint. The silicon crystals are grey to black, needle-shaped or hexahedral (cubic shape). The amorphous phase is a dark brown powder.
Silicon is a semiconductor, its electrical conductivity is much lower than that of metals. It is insoluble in water (except at high temperatures). Silicon has bluish metallic reflections, but is not at all as ductile as metals. There are three natural isotopes of silicon: ²⁸Si (92.18%), ²⁹Si (4.71%) and ³⁰Si (3.12%). There are also unstable artificial isotopes: ²⁵Si, ²⁶Si and ²⁷Si which are senders β⁺, as well as ³¹Si to ³⁴Si which are senders β-.
One of the silicon compounds, silica (silicon dioxide or SiO₂), was already known in ancient times. Silica was considered as an element by alchemists then chemists.
Antoine-Laurent Lavoisier had suspected its existence in 1787, but silicon chemistry began when silicon was first isolated in 1823 by Jöns Jacob Berzelius. It was not until 1854 that Henri Sainte-Claire Deville obtained crystalline silicon.
In addition to the properties of elemental silicon, many silicon compounds have applications. Among the best known: - it silica is found in nature in compact form (pebbles, quartz vein for example), or sand more or less fine. It is also obtained industrially in powder form (synthetic silica). It has many uses: the lens has been made for millennia by melting sand mainly composed of SiO₂ with calcium carbonate CaCO₃ and sodium carbonate Na₂CO₃. Glass can be improved by various additives, Silica sand is one of the components of ceramics, the quartz forms beautiful crystals. It is used as a transparent material, more heat resistant than glass (halogen bulb). It is also much harder to melt and work, silica is used alongside carbon black in the manufacture of energy-efficient tyres ("green" tyres), Very fine silica is used as an admixture for high performance concrete; - ferro-silicon and silico-calcium are used as addition elements in the production of steel or cast iron; - it silicon carbide has a crystal structure similar to that of diamond; its hardness is very close. It is used as an abrasive or in ceramic form in machining tools; - calcium silicate CaSiO 3 is one of the components of cements. - From recent research allow new uses of silicon to be considered.
Silicon does not exist naturally in the free state on earth, but it is very abundant in the form of oxides, for example silica or silicates. Silicon is extracted from its oxide by metallurgical processes, and its purity level depends on its end use. These various considerations have led to silicon being used only in certain types of application. It has been used for a very long time in the form of amorphous silicon oxide (silica or SiO₂) as an essential component of glass. Since the middle of the 20th century, it has had new uses in electronics (transistors), for the production of materials such as silicones or to manufacture photovoltaic solar panels.
To avoid frequent translation errors from English, it should be noted that English silicon means silicon, while silicone is a good match for silicone. On his side, silica is silica.
In cosmetic products Silicon is used indirectly in different forms and in different types of applications. These are mainly: - natural and synthetic mineral silicates, - Colloids, - silica, - silicones, - organic silicon, - SolGel.
We will address these different uses in a series of contributions to come.
◊♦◊♦◊♦◊♦◊♦◊♦◊♦◊♦
Controversy
Then why did carbon take him to Earth? The possibility was raised of a completely different form of life based mainly on silicon and not carbon. This could be based on the fact that silicon is not only tetravalent like carbon, but is likely to form charged and stable penta- and hexa-coordinated complexes. However, it should be noted the extreme difficulty of silicon to form multiple bonds that are necessary for the functioning of exchanges in the cell. Examples of silicon molecules with multiple bonds or different valencies of IV are only possible with particularly complex substituents. At low temperatures, the structure of silicon is very stable and lends itself easily to information storage. But by -220°C the chemical reactions are strongly slowed down. At more reasonable temperatures, the chemical properties of silicon do not allow a molecular structure as varied as that of carbon and information storage weakens. Moreover, silicon is difficult to exchange its electrons, and is not used in exchanges between cells. It often refuses to associate with other atoms. Silicon is also not soluble in water. Finally, it is found in the universe in quantities ten times lower than carbon. Its abundance drops to 7% in the solar system. Certain theories are considering that silicon could have another form of life. Indeed, exobiologists have speculated on the existence of life forms no longer built on carbon, but on an atom with similar properties, silicon. However, this hypothesis has so far been little validated. Thus, for a hundred or so carbon molecules, only a few silicate molecules are found. So the Universe doesn't seem to have had much fun with silicon. For the moment, only science fiction gives life to organisms whose biology differs radically from that of terrestrial beings. .
For more on this subject • http://fr.wikipedia.org/wiki/Silicium • http://www.astrosurf.com/luxorion/bioastro-chimiesi.htm • http://sili.cium.free.fr/ch3.htm • http://www.universalis.fr/encyclopedie/silicium/ • http://www.espace-sciences.org/sciences-ouest/comprendre-le-sable-sa-chimie-ses-applications
| Contribution made by Jean Claude Le Joliff A biologist by training, Jean Claude Le Joliff was a man of R&D for many years. Successively in charge of R&D, then of Research and Innovation in a large French group of cosmetics and luxury goods, and after an experience of setting up a research centre (CERIES), he turned to innovation management. He has also been an Associate Professor at the University of Versailles Saint Quentin (UVSQ) and remains in charge of courses in the framework of several specialized courses: ISIPCA, IPIL, ITECH, UBS, UCO, SFC, etc. He is the founder of inn2c, an R&D and innovation consulting company. Consultant for several international companies, he has actively participated in projects such as Filorga, Aïny, Fareva, and many others. He created the Cosmétothèque®, the industry's first conservatory of crafts and know-how. |