There are several definitions of clays. The term"clay" refers, depending on the case, to a group of mineral species, a family of rocks, a category of soil or a granulometric class. Under this generic name hides in fact a great variety of materials, whose common point is to possess clay minerals, which are, them, of very pre(silicate based) and whose structure gives them, compared to other types of soils or rocks, very specific properties with regard to their interaction with water.
Clay
The clays come from the alteration and degradation of rocks, physical alteration under the effect of temperature variations, and especially chemical alteration in contact with water which allows degradation into very fine particles. For this reason, they are systematically found in soils and surface formations. The conditions in which this degradation took place, as well as its state of progress can explain the great clay diversité́ Mixed with another mineral like calcite, clay will form marl. If it is present alone, the rock will be called argillite. Argillite is a sedimentary rock composed largely of clay minerals, generally more or less hydrated aluminium silicates. For a long time the term"clay" corresponded to all minerals with a size of less than 2 µm in a rock. This particle size cut is inherited from studies carried out by optical microscopy at the end of the 19th century. Today, the name"clay" differs according to the fields of study. In clay science, it does not correspond to a particle size cut, but to minerals. Clay is a mineral with a laminated structure (phyllosilicates) explaining its plasticity, or fibrous (sepiolite and palygorskite) explaining its quality of absorption.
Structure of clay minerals
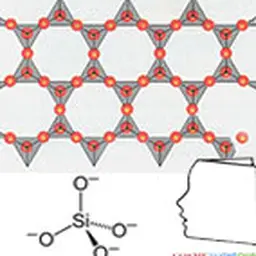
Their structure is characterized by the superposition of sheets composed of tetrahedral or octahedral layers. These are classified into three main families according to sheet thickness (0.7, 1 or 1.4 nm), which correspond to a number of layers of tetrahedral (Si) and octahedral (Al, Ni, Mg, Fe 2+ Fe 3+ Mn, Na, K, etc.).
The interstice, or interleaf space, can contain water as well as cations like K, Na, Ca. This results in variations in the distance between sheets, and thus macroscopic dimensional variations of the clay when it hydrates (dilatation) or dries out (contraction).
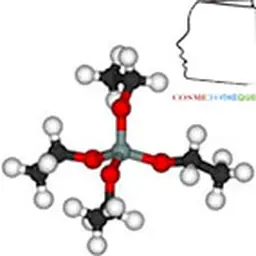
The basic cell of clay minerals is called crystallite. The crystallite (or unité́ structural) is composed of a sheet and an interleaf: each sheet is itself formed by the superposition of two or three crystallized layers (i.e. in which atoms, solid at ordinary temperature, are regularly distributed). There are two types of layers: - the tetrahedral sheets which are arranged in hexagonal meshes and consist of oxygen tetrahedrons surrounding a silicon or aluminium atom ; - Octahedral sheets are composed of octahedrons formed by two oxygen-hydroxyl planes framing larger atoms such as Al, Fe, Mg, Li, etc. The composition of the layer plays a role. For a purely magnesian octahedral layer, for example, consisting of Mg atoms 2+ On the other hand, for a purely aluminous octahedral layer, the swelling capacities are different (see chapter Veegum™).
The interleaf consists of fluid (water) ensuring an electrochemical bond between the sheets. There are different types of interleaf links.
A clay particle thus results from the stacking face to face of some elementary crystallites. The different possibilities of stacking the layers in the sheets, of isomorphic substitutions, of interleaf bonds and finally of spatial arrangement of the crystallites results in the great diversity of structures and properties of the clays.
The electronegativity of the sheets is one of the fundamental characteristics of clays. Substitutions may occur between the different constituent atoms of the tetrahedral or octahedral layer. These substitutions induce a permanent charge deficit, making many clays stable negatively charged species. These charges are compensated by the incorporation of cations within the clay interleaf. Electroneutrality is obtained by adsorption of compensating cations on the surface of the sheets: cations (K+, Na+, Mg2+, Ca2+, Fe2+…) coming from the fluid.
The cations constituting the octahedral sheet induce, according to their valence, a modification of the filling rate of the layer. As a result, some clays have the ability to increase their interfoliary spaces. This property comes from the incorporation of hydrated cations (Na, Ca, etc.) It is the water incorporated via the hydrated cations that allows the crystalline building to swell. The higher the humidity, the greater the swelling.
Finally, the OH - present at the edge of the clays also induce an exchange and adsorption capacity of charged species. However, these hydroxyl groups are pH dependent. This property plays a role in the clay's exchange capacity.
Use of clays in cosmetics The most interesting properties for cosmetic applications are swelling (colloidal suspensions and thixotropic effect) and exchange (complexation capacity). Finally, the absorbent properties complete their interest.
Colloidal suspensions
This is one of the basic properties that leads to the use of clays in formulation. The objective is to control the sedimentation of particulate dispersions, whether pigmentary or not. The special properties of clay minerals are due to their small size, sheet structure and negative particle charge. Together with water, they form non-sedimentary colloidal solutions. These colloids, or colloidal"solutions", are mixtures (liquid, gel) that contain suspended particles. These particles, or colloidal objects, are larger than their constituent molecules (supramolecular size) but small enough to keep the mixture homogeneous.
The stability of a colloidal suspension results from the balance between the attractive interactions and the repulsive interactions that are exerted on the particles. These interactions depend in particular on temperature, pH and dissolved electrolytes. It is obtained by preventing the aggregation of particles from the dispersed phase. In nature, colloids can be either electronegative (clays, humus, iron-silica complex) or electropositive (iron oxides, alumina oxides, starch). For example, around electronegative colloids such as clays, a cloud of positive charges is formed consisting essentially of hydrogen ions (H + or H 3 O + ) and metal cations (Ca ++ Mg ++ , K + Na + Fe +++ or Fe ++ Al +++ ) or ammonia (NH 4 + ). As a result, the swelling clays form non-sedimenting gels of variable viscosity that either give viscosity to the systems or develop non-sedimentation properties. This property is highly sought after for certain formulation systems.
Conversely, the neutralization of negative charges by positive charges allows colloids to agglomerate and form a floc. They say colloids are in a flocculated state. The non-sedimentation property is lost.
These colloidal suspensions can be obtained from hydrophilic colloids such as bentonites or montmorillonites, or from organophilic clays in anhydrous media.
Thixotropy
Thixotropy is a physical property found in certain gels, fluids or fluid mixtures, which have the particularity of seeing their flow properties vary over time. It can be defined as follows: a fluid or material is thixotropic if, under constant stress, its apparent viscosity decreases over time .
This phenomenon is explained by a change in the structure of the fluid when it is sheared. The term thixotropy is used for reversible phenomena. The physical property of thixotropy is therefore: - left to rest for a long time, the thixotropic fluid will restructure, its viscosity increases, - under sufficiently high stress to break the structure formed at rest, the material can flow and destructure, its viscosity decreases with the progress of destructuring.
In formulation, this phenomenon is implemented mainly to manage sedimentation control. It is generated by colloidal systems such as hydrophilic or organophilic clays. It is the basis, for example, of non-sedimenting varnishes and often present in form systems in the make-up world as soon as a pigment suspension needs to be stabilized. It can also be used as part of viscosity regulation for specific questions. It can also contribute to changing the tactile perception of products mainly during application.
Power of absorption and exchange
Another property of clays used in cosmetic products is their absorption and exchange capacity. Most of the clays in suspension carry negative charges. The cations compensating the permanent and variable loads of the clay remain, for the most part, exchangeable. Each clay thus has a CEC (cation exchange capacity) of its own, testifying to their belonging to one of the great families of clay minerals. As an indication, smectites have a much greater exchange capacity than kaolinites. It is this property that is used when clays are used to purify certain systems. From this point of view, they behave as very effective complexing agents.
Absorbent properties
Beyond their water-absorbent properties to form pastes, the specific surface of these materials makes them good absorbents for many substances. Their interest will be, for example, in applications to control the level of sebum in the skin, the absorption of oil to adjust touch or texture, or the development of mattifying properties by absorption of excess fat.
The main colloids
Kaolin
Kaolin is actually a rather special type of silicate. It is a clay which corresponds to the formula: Al 4 [(OH) 8 If 4 O 10 . Depending on the strength of the links between the clay sheets, they may or may not allow water to enter the interfoliary space. According to the classification for certain clays (kaolinites, illites), water cannot engage between the sheets. These are slightly swollen. Thus kaolin, which is a clay, has no swelling character in water.
Use in cosmetics It has interesting complexing properties, for example with respect to calcium, thus significantly reducing water hardness. Some uses in formulation are: - or to be a basic element for powder formulation, - or to be a mattifying agent in certain types of formulations, - or a complexing agent in makeup remover, - or an absorbent agent in mask formulations.
Bentonite, Montmorillonite and others
The alteration and hydrothermal transformation of ash from glass rich volcanic tuffs leads to the neoformation of clay minerals, which are mainly part of the smectite group. The clayey rocks formed are named bentonite after the deposit near Fort Benton, Wyoming, USA. It contains over 75% montmorillonite. The latter was first discovered in 1847 near Montmorillon, in the department of Vienne (France).
Smectites are characterized by a high capacity for adsorption, ion exchange and swelling, as well as by particular rheological properties (thixotropy). In smectites, the weak bond between leaves means that each interleaf space can hydrate. The amplitude of the swelling depends on the initial state, of course, and the stresses applied, nevertheless the water intake can be such that the volume of the material is multiplied by twenty. There are many varieties of this type of clay.
They form colloidal suspensions to stabilize dispersions or emulsions, or adjust the viscosity of different types of formulas. The choice will be made according to the objectives of the formulator.
There are also some specific qualities of clay with a particular provenance. They can also be coloured, like green clay. It is basically clay of the same type as the basic qualities, they can simply be used for particular uses according to needs. They do not have any specific characteristics.
Veegum ®
INCI"Magnesium aluminium Silicate" is a natural smectite type clay that is treated to optimize its performance and purity. Due to their composition, and in particular a higher magnesium content than the usual qualities, these clays are more swellable than some other varieties.
The interest of Veegum® clays as stabilizers and rheological agents is due to their colloidal structure in water. Each smectite particle consists of thousands of submicroscopic platelets stacked in sandwich mode with a layer of water between each. Their advantage comes from their ability to generate gels at lower concentrations. Indeed, in the formulation of make-up products, the mattifying effect of clays can be a handicap. Obtaining stable colloidal gels and suspensions at lower clay concentrations is of major interest. These are good choices for the formulator to stabilize emulsions and optimize suspension flow properties.
The clay veins used to make Veegum® products are mined in Nevada, Arizona and California. There are many varieties corresponding to various uses. - VEEGUM R : the original and most widely used grade in all types of cosmetic and personal care products. - VEEGUM Ultra: a unique smectite acid that produces dispersions in the pH range of 4.2 to 5.2 for optimal compatibility with a naturally acidic skin pH. - VEEGUM CH : a natural smectite for use in high pH personal care preparations such as hair relaxers, depilatories, and waving products. - VEEGUM HV a higher viscosity grade used in suspensions, topical emulsions and other personal care products. - VEEGUM K: provides the right suspension with low viscosity. - VEEGUM F : an ultrafine powder perfectly adapted for uniform dry mixtures, very useful for direct compression tablets. - VEEGUM HS: offers maximum electrolyte stability and minimal acidity. It is used in pharmaceutical suspensions of acid pH, in hair care formulas containing conditioning agents and face masks. - VEEGUM D: an excellent toothpaste binder which guarantees a stable paste whatever the temperature. - VEEGUM Plus : a mixture of smectite and CMC with effective thickening value at very high yield. - VEEGUM PRO : a chemically modified smectite with greater thickening efficiency for personal care.
Use of smectites in cosmetics Depending on needs, these substances can be used for different functions in systems containing water: gelling agent, viscosity regulation, development of colloidal suspensions to control sedimentation, texture agent, mattifying agent, absorption.
Organophilic clays
A particular variety of silicates derived from natural clays is referred to as organophilic clay. The first published results on organophilic clays appeared in the early 1960s. This work followed the need to develop depollutants for environmental systems capable of absorbing substances with low polarity (oils and lipophilic pollutants). A new family of microporous solids at porosité́ controlled similar to zeolites, commonly called organophilic clays or bridged clays or pillar clays (AP) has been developed in this sense.
The principle is based on the fact that clay minerals of a hydrophilic nature can be rendered organophilic by exchanging their interfoliate cations with cationic surfactants, such as ammoniums. This modification makes it possible to obtain materials that can be used in different applications such as adsorption of organic pollutants.
Alkylammonium salts are the most commonly used for the preparation of organophilic clays. It is these substances that have thus been developed and widely used in formulation, either to manage the viscosity or rheology of anhydrous systems, or to manage non-sedimentation of particular dispersion. Among the first applications were nail polishes.
These products are particularly known under a common name which is Bentone™, but there are other trade names. These products are obtained from natural clays, bentonite or montmorillonite, from which mineral cations are extracted for their replacement by organic cations. The INCI names of the main specialties are: Stearalkonium hectorite, Disteardimonium hectorite.
The choice of these substances will depend on the polarity of the medium in which they are used. Graphs allowing these choices are available to formulators.
It is common practice to use these colloids in the form of pre-dispersions, described as Master Gel. This is due to the difficulty of dispersing these colloids precisely in organic media. This dispersion operation significantly improves rendering. Moreover, their use presupposes the joint presence of an"activating" agent which is a highly polarity substance. When colloids are used in powder form, the presence of a small amount of water may be sufficient as an activator. When used in the form of Master gel anhydrous, the activating agents are commonly propylene carbonate, propylene glycol or ethanol depending on the qualities and media in which these colloids are dispersed.
There are many Master Gel specialities around these colloids. We can quote in a non-exhaustive way: - C13 Isoparaffin, Disteardimonium hectorite, Propylene carbonate ; - Octyldodecanol, Disteardimonium hectorite, Propylene carbonate ; - Helianthus annuus seed oil, Disteardimonium hectorite, Propylene carbonate, Tocopherol ; - Isohexadecane, Disteardimonium hectorite, Propylene carbonate ; - Mineral oil, Disteardimonium hectorite, Propylene carbonate ; - Olea europaea fruit oil, Stearalkonium hectorite, Propylene carbonate ; - Cyclopentasiloxane, Disteardimonium hectorite, Propylene carbonate.
Use in cosmetics Specific to anhydrous media, these substances develop properties close to hydrophilic colloids: gelling agent, viscosity regulation, development of colloidal suspensions to control sedimentation, texture agent.
Frametime ™
It is a speciality developed and patented by Ephyla. Resulting from the alloy between a natural clay bentonite type and a biomolecule, this specialty allows the development of several types of application: - Viscosant first, - but also emulsifying, in particular systems based on"cold process" by simple mechanical agitation, - colloid-based encapsulation, - emulsifying in Pickering type systems.
There are several varieties: - Frametime CX, INCI : Bentonite & Xanthan gum & Citric acid ; - Frametime CXG, INCI : Bentonite & Xanthan gum & Citric acid & Sodium stearoyl glutamate, which stabilizes a cold emulsion by simple mechanical agitation ; - Frametime CCL, INCI : Bentonite & Chitosan & Lactic acid ; - Frametime CHA, INCI : Bentonite & Citric acid & Sodium hyaluronate.
Examples of formulations containing functional silicates
Beauty mask AQUA (WATER) - KAOLIN - GLYCERIN - OLUS OIL (VEGETABLE OIL) - PROPYLENE GLYCOL - DICAPRYLATE/DICAPRATE - CETEARYL ALCOHOL - GLYCERYL STEARATE - VITIS VINIFERA (GRAPE) SEED OIL - NEOPENTYL GLYCOL - DIETHYLHEXANOATE ETHYLHEXYL PALMITATE - CAPRYLIC/CAPRIC TRIGLYCERIDE - BENTONITE - CET ALCOHOL - CETETH-20 PHOSPHATE - MAGNESIUM ALUMINUM SILICATE - CI 77891 (TITANIUM DIOXIDE) - BENZYL ALCOHOL - DICETYL PHOSPHATE - TOCOPHERYL ACETATE - CITRUS AURANTIUM BERGAMIA (BERGAMOT) FRUIT OIL - HYDROGENATED VEGETABLE OIL - CAPRYLYL GLYCOL - LAVANDULA ANGUSTIFOLIA (LAVENDER) OIL - LINUM USITATISSIMUM (LINSEED) SEED EXTRACT - EUPHORBIA CERIFERA (CANDELILLA) WAX - DEHYDROACETIC ACID - CUPRESSUS SEMPERVIRENS OIL - SALVIA SCLAREA (CLARY) OIL - SODIUM HYDROXIDE - COMMIPHORA MYRRHA OIL - SANTALUM ALBUM (SANDALWOOD) OIL- SODIUM PHYTATE - ANTHEMIS NOBILIS FLOWER OIL P- ETROSELINUM CRISPUM (PARSLEY) SEED OIL - ZINC PCA - SILICA - LINALOOL.
Face care cream AQUA (WATER/EAU) - HEPTYL UNDECYLENATE - CAPRYLIC/CAPRIC TRIGLYCERIDE - GLYCERIN - CETYL ALCOHOL - CETEARYL OLIVATE SORBITAN OLIVATE ALCOHOL - BOROJOA PATINOI PULP EXTRACT - HYDROLYZED HYALURONIC ACID - ACMELLA OLERACEA EXTRACT - HELIANTHUS ANNUUS (SUNFLOWER) SEED OIL -TOCOPHEROL - ULVA LACTUCA EXTRACT - BENTONITY - PALMITOYL TRIPEPTIDE-38 DIPEPTIDE -DIAMINOBUTYROYL BENZYLAMIDE DIACETATE - MONTMORILLONITE - XANTHAN GUM - FRAGRANCE - PHENETHYL ALCOHOL.
Foundation foundation AQUA (WATER) - ETHYLHEXYL PALMITATE - TALC - NEOPENTYL GLYCOL DIETHYLHEXANOATE - CYCLOPENTASILOXANE - SILICA - CAPRYLIC/CAPRIC TRIGLYCERIDE - ETHYLHEXYL METHOXYCINNAMATE - HAMAMELIS VIRGINIANA (WITCH HAZEL) WATER - PROPYLENE GLYCOL - GLYCERIN - PEG-30 DIPOLYHYDROXYSTEARATE - PEG-30 HDI/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER - ALUMINUM HYDROXIDE - GLYCYRRHIZA GLABRA (LICORICE) ROOT EXTRACT - BUTYLENE GLYCOL - ALCOHOL - PHENOXYETHANOL - SILICA DIMETHYL SILYLATE - TRIBEHENIN - METHYLPARABEN - DISTEARDIMONIUM HECTORITE - PROPYLPARABEN - PERFUME (FRAGRANCE) - BHT - LAMINARIA DIGITATA EXTRACT - ASCORBYL PALMITATE - GLYCERYL STEARATE - DISODIUM STEAROYL GLUTAMATE - CITRIC ACID - SODIUM HYALURONATE - BUTYLPARABEN - ETHYLPARABEN - CI 77891 (TITANIUM DIOXIDE) MICA.
Nail polish BUTYL ACETATE, ETHYL ACETATE, NITROCELLULOSE, PHTHALIC ANHYDRIDE/TRIMELLITIC ANHYDRIDE/GLYCOLS COPOLYMER, ISOPROPYL ALCOHOL, ISOBUTYL ACETATE, ACETYL TRIBUTYL CITRATE, STYRENE/ACRYLATES COPOLYMER, STEARALKONIUM HECTORITE BENZOPHENONE-1, TRIHYDROXYPALMITAMIDOHYDROXYPROPYL MYRISTYL ETHER, ALGAE EXTRACT, DIMETHICONE, PHOSPHORIC ACID. May also contain (+/-): CI 12085 (RED 36), CI 15850 (RED 7 LAKE), CI 15880 (RED 34 LAKE), CI 15985 (YELLOW 6 LAKE), CI 19140 (YELLOW 5 LAKE), CI 42090 (BLUE 1 LAKE), CI 47005 (YELLOW 10 LAKE), CI 75170 (GUANINE), CI 75470 (CARMINE), CI 77000 (ALUMINUM POWDER), CI 77007 (ULTRAMARINES), CI 77163 (BISMUTH OXYCHLORIDE), CI 77491, CI 77492, CI 77499 (IRON OXIDES), CI 77510 (FERRIC FERROCYANIDE), CI 77742 (MANGANESE VIOLET), CI 77891 (TITANIUM DIOXIDE), MICA
For more on this subject
•
http://fr.wikipedia.org/wiki/Argile
•
http://www.universalis.fr/encyclopedie/colloides/
•
http://fr.wikipedia.org/wiki/Colloïde
•
https://www.u-picardie.fr/~beaucham/mst/argiles.htm
•
http://www.ecosociosystemes.fr/colloides.html
•
http://fr.wikipedia.org/wiki/Talc
•
http://fr.wikipedia.org/wiki/Mica
- How to structure the oily phases - Julie Saintecatherine- Cosmetic Expression October 2014 - 64-68
- Smectite organoclay chemistry. - Dwaine Siptak - The chemistry and manufacture of Cosmetics - Vol III Third Edition 2002 - 845-855
- Design of cosmetic products: formulation - Anne-Marie Penssé L'Héritier - Lavoisier 2014.
- Thesis for obtaining the title of Doctor of the University of POITIERS - Factors determining the organization and rheology of clay/water systems for Smectites suspensions - Sandrine PAUMIER .
- Thesis presented in view of obtaining the title of Doctor of the École Polytechnique: Modélisation du gonflement des clailes et de ses effets sur les ouvrages de stockage souterrain. Jérôme Gaombalet
| Contribution made by Jean Claude Le Joliff A biologist by training, Jean Claude Le Joliff has been a man of R&D for many years. Successively in charge of R&D, then of Research and Innovation in a large French cosmetics and luxury group, and after an experience of setting up a research centre (CERIES), he turned to innovation management. He has also been an Associate Professor at the University of Versailles Saint Quentin (UVSQ) and remains in charge of several specialized courses: ISIPCA, IPIL, ITECH, UBS, UCO, SFC, etc. He is the founder of inn2c, an R&D and Innovation consulting company. Consultant for several international companies, he has actively participated in projects such as Filorga, Aïny, Fareva, and many others. He created the Cosmétothèque®, the industry's first conservatory of crafts and know-how. |