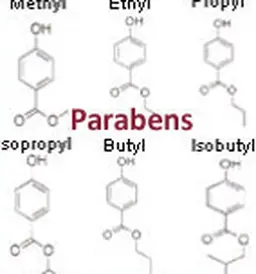
Restriction had been on the agenda for several months following the converging opinions of the SCCS, the European Scientific Committee in charge of assessing the safety of cosmetic ingredients. It has now been given the force of law with the publication of regulation 358/2014, which modifies the Cosmetics Regulation. Several parabens are thus banned, and the use of triclosan is restricted.
Regulation 358/2014 is dated April 9, 2014 and has been published in the Official Journal of the European Union on April, 10. It amends Annex II (substances prohibited in cosmetic products) and Annex V (list of preservatives allowed) of the Cosmetics Regulation 1223/2009.
It is binding and directly applicable in all Member States of the European Union.
Less triclosan
Triclosan is currently allowed at a maximum concentration of 0.3% as a preservative in cosmetic products.
The experts' scientific opinion
The European experts have published several opinions on triclosan. Their conclusions:
• the use of triclosan as a preservative at the current maximum concentration limit of 0.3% in all cosmetic products is not safe for the consumer because of the magnitude of the aggregate exposure,
• its use at a maximum concentration of 0.3% in toothpastes, hand soaps, body soaps/shower gels and deodorants, face powders and blemish concealers is safe.,
• other uses of triclosan in nail products where the intended use is to clean the fingernails and toenails before the application of artificial nail systems at a maximum concentration of 0.3% and in mouthwashes at a maximum concentration of 0.2% are safe for the consumer.
The new regulation
Annex V, …