There are many ways to moisturize the skin, or more accurately prevent it from becoming dehydrated. As a result, there are many substances with these properties. Moreover, skin hydration remains one of the main concerns of the cosmetics industry under pressure from users. Close-up on osmolytes, slowers of water loss.
In a previous contribution we described several groups of substances used for hydration, including osmolytes. Osmolytes are small molecules soluble in intracellular solution, found in several species, and play a role in combating environmental stress in living organisms. They make it possible to balance the osmotic pressure of the various tissue compartments. They can be subdivided into several categories. Today we are trying to better understand their modes of action and what they are thanks to a contribution from Olga Gracioso who has worked on this issue. Thanks to her and Sederma.
Jean Claude Le Joliff
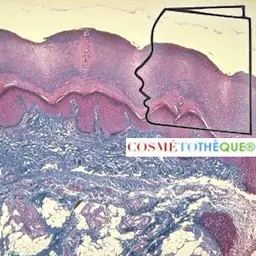
The skin perspires water 24 hours a day; this aqueous efflux from the dermis towards the epidermis creates a local cutaneous hydration gradient varying from 70% in the dermis to only 10-12% in the epidermis and, by this route, approximately 80-180 ml/m 2 /day are perspired.
For a well-hydrated skin, this outgoing flow must be perfectly controlled: this is the role played by the epidermal barrier of the stratum corneum . To reach the skin surface, perspiration water passes through two different pathways: the peri-cellular pathway (concerns 1/3 of tissue water) but also the intracellular pathway via specialized channels: aquaporins (2/3 of water is intracellular).
Aquaporins AQP3 of the skin are specific membrane protein channels of water (and glycerol), which are found in the keratinocytes of the basal layer and which allow the transit of water and glycerol from the dermis to the epidermis.
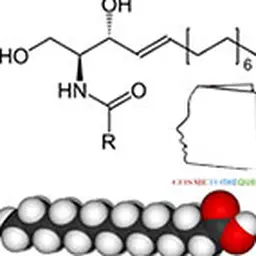
Glycerol also plays an important role in the upward circulation of water: in knock-out mice with AQP3, the water and glycerol content of stratum corneum is deficient but normal hydration can be restored either by intraperitoneal injection of glycerol or by topical application of glycerol. The skin then covers its capacity (measured by corneometry) and flexibility (measured by cutometry), (Hara et al, 2002 and 2003). Thus, glycerol, by binding locally with water molecules, contributes to the intrinsic water retention capacity of the epidermis (or"Water-Holding Capacity" in English).
Thus, we can better imagine how the skin maintains its hydration, thanks to the coexistence of three phenomena: - dynamic water circulation via aquaporins and intercellular spaces towards the surface, - water retention by a stratified and well-structured corneocyte barrier that limits perspiration, - and finally the presence of polyols which, through hydrogen bonds, partially and locally capture water molecules in transit.
However, if the skin tends to dry out, the use of moisturizing cosmetic products (about 40% of the market) is a way to compensate for the deficiencies of the phenomena described above. Glycerin hygrophilic, ceramides to repair the skin barrier, many strategies have been developed to optimize skin hydration. We will describe here an adaptive strategy of nature, osmoregulation, which allows water to pass through the skin, from the surface to the epidermis and, more slowly, to the dermis.
Osmolytes: nature's adaptive strategy
Osmolytes were discovered relatively late in the early 20th century in marine organisms and were long considered unique to these species. Today, however, we know that they are universal: developed, selected and preserved by nature during evolution throughout the living kingdom. They allow organisms to survive or cope with sudden and significant changes in their living environment.
Indeed, each cell needs, to maintain its activity, to control and regulate its volume and its intracellular water (70% water in the cells). When the sudden changes in available water are of short duration, ionic exchanges (incoming or outgoing flow of inorganic ions) allow rapid rebalancing, but, when the situation persists, the cells set up a more advanced system of protection by playing on the osmolytes. In case of hyper-saline stress for example, a cyanobacterium will synthesize and accumulate osmolytes which will slow down water loss and physically occupy space by recreating an environment favourable to the activity of intracellular proteins.
The definition of an osmolyte is as follows: "Cell solute mobilized by cell significantly to maintain or adjust osmotic pressure and cell volume." . (Yancey, 2005).
Osmolytes have been grouped into four classes (Yancey, 1994 and 2005): - amino acids and their derivatives, - methylamines, - carbohydrates or polyols, - urea.
As a result, many osmolytes are common to different species. Moreover, they are always a mixture. Thus, each species presents its specific cocktail, with variable proportions according to the different categories of osmolytes. However, uncharged carbohydrates are certainly the most universally used compounds. In the end, no cell, bacterial, vegetable or animal, composed of 70% water, can survive and adapt to water variations without recourse to osmolytes.
In our body, this is particularly well illustrated at the level of our kidney cells which must function with an environment rich in salt and urea during the formation of urine from blood: sorbitol, myoinositol, GPC and betaine are used by the cells of the kidney cortex. On the other hand, for our nerve cells, the adjustment to variations in blood composition is done rather by taurine.
Skin and osmotic shocks
Osmotic shocks induced on human keratinocytes are managed, according to the work of Warskulat et al, 2004, by the cocktail betaine/myoinositol/taurine. Thus Warskulat (2007) demonstrates that osmolytes have a protective, anti-drying and anti-apoptosis UV effect.
Curiously, glycerol does not appear, in the few publications on human skin, as an osmolyte (i.e."compound mobilized significantly by the cell to maintain or adjust osmotic pressure and cell volume"). One might consider glycerol important for the hydration of human skin, but in a normal situation without stress, it is somehow a"resident moisturizer". All these data lead us to look for an osmolyte different from glycerol to regulate skin hydration.
Platymonas subcordiformis
This unicellular alga is interesting because of its habitat: it lives in estuaries and is therefore confronted every six hours with a change in salinity. The examination of the osmolytes of Platymonas reveals, as usual, the presence of a mixture of carbohydrates and amino acids, but it is an original mixture. Erythritol is associated with mannitol and homarin with betaine (Dickson et al, 1986). Erythritol and homarin are interesting osmolytes because of their properties. Homarin is a cyclic osmolyte often found in phytoplankton and marine invertebrates such as gorgonians.
In a hyper-saline shock, Platymonas reacts with a neosynthesis of zwitterions: homarin intervenes, with a higher speed and quantity compared to betaine, allowing this algae to resist salt concentrations of 1000 mol/m³ (1 M!!!!). This ability of homarin to contribute durably to the osmotic balance comes from its cyclic structure which makes it a more stable osmolyte.
Polyol of 4 carbons, erythritol is the higher homolog (four carbons) of glycerol (three carbons). Few data are available in the literature concerning its properties as an osmolyte. However, its presence, as well as the increase in its production, under stressful conditions, in yeasts and algae has been reported. Moreover, it seems to be characterized by very good permeation.
Inspired by the example of the Platymonas Sederma has developed a cosmetic composition to regulate skin water imbalances. This product, whose trade name is Aqualance ® consists of the following compounds: - Erythritol, for its permeation properties, which can increase cutaneous water holding capacity, - homarin, as a more stable cyclic zwiterrion than betaine, to counterbalance ion imbalances. The couple zwiterrion-polyol thus forming an osmo-balancing entity.
The effectiveness of this product has been demonstrated. As an example, we can cite this result: 20 women aged 23 to 67 years received a bi-daily gel on their legs containing 3 % Aqualance® or placebo. Skin hydration measurements were taken after 1h to 7h, 24h and 8 days at Corneometer® (Courage&Khazaka), MoistureMeter-D™ (Delfi n) and in the near infrared. The three tests revealed that the hydration kinetics in the different skin layers are complementary to the skin's water gradient. The water content stabilizes after 1h in the stratum corneum After 2 hours in the epidermis and after 24 hours in the dermis.
The effectiveness of this product, called Aqualiance™, has been demonstrated by tests in vitro and in vivo available on Sederma's website (connect to the extranet to access it) or on request by sending an email at this address .
References - HARA M, MA T, VERKMAN AS, 2002 - Selectively reduced glycerol in skin of aquaporin-3-deficient mice may account for impaired skin hydration, elasticity, and barrier recovery. J. Biol. Chem. 2002 Nov 29; 277(48):46616-21. - HARA M, VERKMAN AS, 2003 - Glycerol replacement correct defective skin hydration, elasticity, and barrier function in aquaporin-3-deficient mice. Proc. Natl. Acad. Sci. U S A. 2003 Jun 10; 100(12):7360-5 - YANCEY, 1994 - Cellular and molecular pharmacology of cell volume regulation. Edt Kevin Strange, (5):86-90 - YANCEY PH, 2005 - Organic osmolytes as compatible, metabolic and counteracting cytoprotectants in high osmolarity and other stresses. J. Exp. Biol. 2005 Aug; 208(Pt 15):2819-30 - WARSKULAT U, BROOKMANN S, REINEN A, HÄUSSINGER D, 2007 - Ultraviolet B radiation induces cell shrinkage and increases osmolyte transporter mRNA expression and osmolyte uptake in HaCaT keratinocytes. Biol. Chem. 2007 Dec; 388(12):1345-52. - WARSKULAT U, REINEN A, GRETHER-BECK S, KRUTMANN J, HÄUSSINGER D, 2004 - The osmolyte strategy of normal human keratinocytes in maintaining cell homeostasis. J Invest. Dermatol. 2004 Sep; 123(3):516-21
| Contribution made by Olga Gracioso Olga is Marketing Director of Sederma, which she joined in 2000. After studying biology and biochemistry, she obtained a Master in Cosmetology at the Faculty of Pharmacy of the University of Paris 11. She began her career in 1997 at Bourjois/Chanel in the evaluation laboratory, then turned to the world of cosmetic ingredients. Its dual scientific and marketing skills enable it to envisage consumer benefits adapted to the needs of different markets based on biological mechanisms of action. It has successfully launched over eighty active ingredients for the beauty industry. She is also a member of the French Cosmetology Society. |
| About Sederma Sederma is a company specialized in the development of active ingredients and the creation of innovative concepts for the cosmetics industry. Its know-how is based on the mastery of biotechnologies, fine chemistry and plant extraction. Founded in 1964, Sederma joined the international Croda group in 1997. The company has a unique range of assets. Sederma also offers a whole series of ingredients from plant cell culture (IRB acquisition by Croda). Equipped with a modern and high performance testing laboratory, Sederma conducts studies in vitro , ex vivo and in vivo to demonstrate the effectiveness of its products. The production and quality control laboratory are also equipped with sophisticated equipment. Sederma is committed to a continuous process of improving the quality of its products and services. In May 2014, Sederma was recognized by Frost & Sullivan as the European Company of Cosmetic Active Ingredients of the Year and in April 2015, Sederma's Matrixyl® received the 25 Years of Innovation Award. |