In a previous contribution, we put into perspective the different fields of use of talc in cosmetic formulations. We take here a publication by Jean-François Robert, who spent most of his professional career in talc. His knowledge of talc mineralogy and geology is unparalleled. He retired in July 2016 after a 35-year career. We thank him for this contribution. Jean Claude Le Joliff
Talcum powder: a brief introduction
The term"talc" meets several definitions: it is first, for the general public, a white powder used for body care (baby powder). But it is also, for the geologist, a mineral and by extension, for the operator, the rock,"ore", from which talc is produced. In the profession, talc is an industrial mineral which owes its multiple applications to specific mineralogical properties.
The mineral has been known and used since ancient times, either in powder form (cosmetics or various fillers) or in its raw state as an ornamental stone that can be carved. For example, researchers have shown that talc is used in the composition of paintings in the Lascaux cave ! Depending on its purity or agglomeration state, talc is known as steatite (massive form), or soapstone for some varieties of impure talc (derived from ultramafic rocks that can be sawn and used as ornamental or refractory stone).
The mineral
Talc belongs to the phyllosilicates family, which includes micas, chlorites and certain clays. The minerals of this family are characterized by the remarkable property of being cut into thin sheets in the manner of mica.
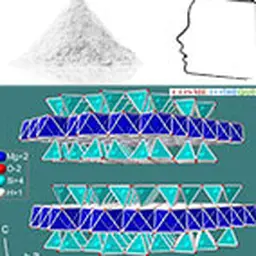
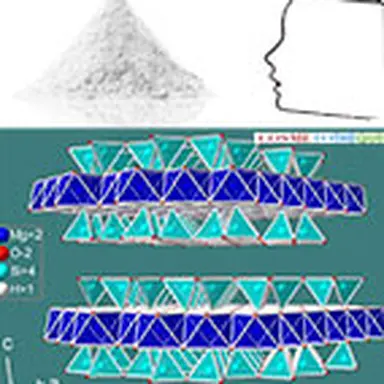
Talc crystallizes in the monoclinic system. At the molecular level, the elementary building is formed by a layer of brucite Mg(OH)₂, interlayered between two siliceous sheets made up of tetrahedrons of type SiO₄. In the primitive pattern, the internal bonds between the layers are both extremely narrow (the brucite layer shares the oxygen sites of the tetrahedral tops of the siliceous layer) and almost completely compensated, which explains why the mineral is electrically neutral. On the other hand, no connection, other than that of Van der Waals, links the elementary motifs together. The specific properties of the mineral, in particular its surface properties (talc is hydrophobic, lipophilic), its soft and smooth touch, its high chemical inertia and its lamellarity, derive from this elementary structure.
Its chemical formula Mg₃ (Si₄ O₁₀) (OH)₂ corresponds to that of a hydrated magnesium silicate with the following theoretical composition : SiO₂ : 63.27% ; MgO : 31.88% ; H₂O : 4.75%. The mineral can admit iron (up to 5%) in its network to replace magnesium. Note that talc has a twin sister with an identical molecular structure: the phyrophyllite (hydrated aluminum silicate) which can in some cases be used in the same applications as talc such as l ’ agalmatolite in Brazil.
The hardness of talc, in the Mohs scale, defines it as the most tender mineral, which is only partially true. In reality, this low hardness is only apparent since it is due to the absence of bonds between the elementary lamellae of the mineral which slide on each other at the slightest stress, giving the impression of softness.
The density of talc (2.65), close to that of other silicates, is not at all specific. Its mass colour is white, greenish or pink when pure. However, talc can have different colours depending on the different impurities that accompany it, such as graphite which gives grey or black talc varieties. In all cases, the powder remains white. Carried at 1000°, talc loses its water constitution and is transformed into enstatite Mg₂ (Si₂ O₆), anhydrous mineral of the family of pyroxenes.
Talc is, by definition, a lamellar mineral. But this property is more or less developed depending on the original deposit. There are microcrystalline talc, i.e. compact and massive varieties in which the lamellarity is practically not detectable whatever the scale of observation, other"microlamellars" where it is expressed in the powder after micronisation, and finally other"macrolamellars" where it can be observed on the macroscopic sample. Lamellarity is sought in many applications for its barrier effect (impermeability) or reinforcing effect (improvement of mechanical characteristics).
In nature, talc is often accompanied by chlorite, an aluminosilicate of magnesium, iron and aluminum, which has similar properties and comparable deposit conditions.
Deposit method and training conditions
Talc is a common mineral in metamorphic rocks. It is characteristic of the"green shale" facies where it appears in talc-schist. However, massive concentrations pure enough to give rise to economic exploitation are rare and in principle of limited size.
Talc deposits always result from the transformation of pre-existing rocks, either magnesian (dolomites, magnesites or serpentinites) or siliceous (quartzites or sandstone pelites), under the effect of hydrothermal circulation which convey one or more of the components necessary for the formation of the mineral, namely : MgO, SiO₂, CO₂ and H₂O. The conditions that govern the formation of the mineral are those of a regional metamorphism of low intensity, i.e. temperatures in the order of 350° to 400°. Finally, the mineralizations thus formed retain some of the physical, chemical or structural characteristics of the original source rocks (see table below).
Generally, tectonics controls the spatial distribution of talcous mineralization and its relationship to host rocks; its role is crucial in the genetic process with the creation of porosity (fracturing and micro-fracturing) that allows fluids to circulate and penetrate the rocks. As for the mineral's crystallinity, in particular its degree of lamellarity, it is determined by the pressures and deformations it has undergone during and after its genesis.
Talc deposits are generally classified according to the nature of the rocks from which they are derived, thus distinguishing those resulting from the transformation: 1) of magnesite source rocks of a carbonate nature (dolomites or magnesites), transformed by siliceous metasomatosis, 2) of siliceous source rocks such as quartzites, for example, transformed under the effect of magnesite metasomatosis; note that in the presence of silico-aluminous rocks (e.g., sandstone or granite), chlorite is added to talc in the reaction paragenesis,
3) of magnesite source rocks of ultrabasic origin like serpentinites under the effect of massive occurrences of CO₂ (carbonization), the original serpentinite is broken down into two mineralogical phases talc and magnesium carbonate (magnesite) with incidentally residual metal ores (magnetite, chromite, various sulphides of iron, nickel and cobalt). In the presence of aluminous minerals in source rock, chlorite may also be present. It should be noted that in this type of deposit, the proportion of talc disseminated in the rock does not exceed 50%.
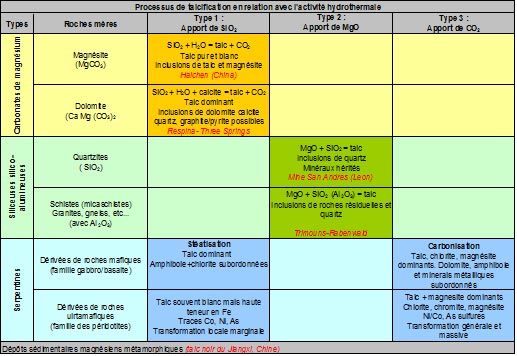
While the first two forms (types 1 and 2), which account for the largest share (>80 %) of world production, can give rise to concentrations pure enough to be exploited as they are, talc/carbonate deposits, derived from serpentinites (type 3) in which the talc content does not exceed 50 %, require wet treatments such as flotation for the production of pure talc powder (see Figure 2 below).
Finally, there are deposits of sedimentary origin which present themselves as inter-stratified layers in series of various natures, often carbonated, and which undoubtedly result from the diagenetic evolution of magnesian sediments (deposits of"sediments"). black talc" Jiangxi, China).
Industrial production
Talc is generally mined in open-pit quarries, more rarely (at least in the West) in underground mines. For pure talc deposits, selective extraction is generally supplemented by manual or mechanical sorting which leads to the production of different grades, separated according to mineralogical criteria, purity, colour, whiteness, granulometry, which will be the basis of the range of commercial products.
The mixed talc carbonate ores of type 3 deposits must first undergo wet flotation enrichment to achieve the same concentration level (see figure opposite).
Talc is a natural product whose treatment is usually limited to drying, grinding and packaging. Exceptionally, it may include surface treatment (silane, amine) to improve its properties, or even calcination. The most widespread grinding technique for talc is that of pendular grinding (Raymond type), which makes it possible to obtain fineness of the order of 40 µ (qualified as standards) with a delaminating effect, which reinforces the natural properties of the mineral. Finer products (30 µ) can be obtained either by selecting standard powders or after micronisation (impact micronisers) and selection (15-30 µ). Compressed air or steam (jet mill) grinding can achieve fineness between 3 and 15 µ. Finally, wet grinding techniques with a delaminating effect make it possible to obtain products with a specific high surface area (HAR).
Once ground, the powders are packaged according to their use and the customer's request. Very fine talc with very low bulk density in powder form is often densified (compacted) into granules to facilitate handling, storage and transport. There are several forms of commercial products: loose powder and granulated products that can be delivered in bags, big bags or in bulk (tanks), and finally slurries, dispersion of powder in a medium in liquid form (in tanks).
The commercial qualities are defined by their degree of fineness, expressed in average particle diameter or particle size cut (top cut), their shape coefficient (lamellarity), their mineralogical purity (chemical analysis) and their whiteness, which is an important qualitative parameter. The following whiteness levels can be synthetically distinguished: - industrial products: bl Y > 75 to 83, - white products: bl Y > 83 to 88, - extra white products: bl > 88 to 95.
Note that the whiteness increases with the fineness of the powder.
Logistics
Talc is an industrial mineral whose worldwide resources are limited and scattered in rather small deposits that are often difficult to rationalize. Minerals remain a heavy commodity: if pure, white qualities, not very competitive and driven by lucrative markets such as the automobile, can reach high prices that allow them to travel, mid-range products must be valued by specific treatments for regional distribution, while low-end grey products competing with other commodities interest only a local market.
Applications and markets
Talc is a filler functional, including industrial applications arise from physical and chemical properties. The range of applications is extremely wide. Moreover, it is not fixed, but on the contrary it is constantly evolving and has had to adapt to the needs of the market and keep pace with technological change. Thus, in addition to its well-known role in cosmetic powders, the traditional applications of talc were mainly, until recently, the paper filler and the carrier of phytosanitary products applied in powder form for agricultural and viticultural treatments. These two functions have nowadays practically disappeared. Indeed, if talc, because of its chemical inertia, was for a long time the unavoidable filler when the manufacture of paper required an acid medium, the technological progress which allowed the adoption of the neutral medium at the beginning of the Eighties in fact removed the decisive technical advantage of talc in this application and led to the development of cheaper alternative fillers such as carbonates. Similarly, in agriculture, the progress of wet processing methods to the detriment of powders has rendered the role of talc inoperative. In parallel, other applications have been developed (and this is the role of the R&D/A function) to replace those that were becoming obsolete, in new markets with higher added value.
Today the main application segments are: - the field of body care and cosmetics, its uses in relation to the surface properties of the mineral, such as the retention of perfume, its softness to the touch, its hydrophobic character ; - the pharmacy where talc is the excipient par excellence, neutral, chemically inert and perfectly harmless; by its lubricating properties (lipophilic), it helps to press tablets ; - in paint, in the decorative paint segment, it is used in all types of formulas thanks to its lamellarity and covering power, as a functional filler, opacifier or matting agent; for very white varieties, it acts as a titanium extender; in industrial paints (anti-corrosion), talc is used for the barrier effect provided by its lamellarity ; - in plastic, its lamellarity combined with its thermal inertia contributes to improving the mechanical properties (rigidity, impact resistance) of reinforced polymers ; talc is increasingly used in the automotive industry (dashboards, trim, bumpers, etc…) where it contributes to reducing the weight of vehicles and it is interesting to note that a modern car contains an average of 15 kg of talc ! it is also used as a functional filler in polypropylene (white electrical appliances), packaging, tubes and profiles..; - in stationery, although talc has lost some of its preponderant place in the field of filler in acid environments, it is still used as a functional filler in addition to other minerals (carbonates, kaolins) for its surface properties and its lamellarity ; it retains a predominant role in the"pitch and sticky" application, i.e. as a passivating agent for residual resins during pulp production and the processing of recycled paper, thus increasing the productivity of paper machines; finally, in coating, it contributes to improving the surface aspect of the sheet and its printability; - in the rubber industry, it is a reinforcing filler used to improve material flow during the manufacture of moulded or extruded parts and fire resistance properties. It is used in tyres to increase impermeability (barrier effect); - ceramics, the Talc applications are more similar to the function of an ore in the sense that, mixed with the other constituents, it is responsible for adding magnesium to the formula; in traditional ceramics, magnesium plays a melting role that makes it possible to lower the firing temperature, whereas in technical ceramics (cordierite), it is a physico-chemical catalyst. In the case of chlorite ores, aluminium is added to magnesium; - Talc is also used as an antimotant and lubricant in the fertilizer industry, animal feed and human food.
Talc in France: Luzenac
 Located 130 km as the crow flies south-east of Toulouse in the Ariège Pyrenees, near the village of Luzenac, the Trimouns deposit has been mined in the open air since the middle of the 19th century. It is one of the largest deposits in the world: it is estimated that it has already produced nearly 20 million tonnes of market talc.
Located 130 km as the crow flies south-east of Toulouse in the Ariège Pyrenees, near the village of Luzenac, the Trimouns deposit has been mined in the open air since the middle of the 19th century. It is one of the largest deposits in the world: it is estimated that it has already produced nearly 20 million tonnes of market talc.
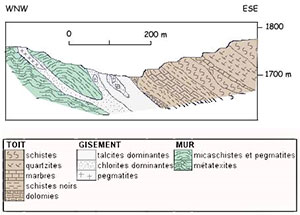
The deposit At the Trimouns Pass, mineralization is located along the eastern edge of the St-Barthélemy massif, along a major and deep tectonic contact whose play led to the overlap of the Paleozoic cover weakly metamorphic on the crystalline basement. The talco-chlorite mineralization is inclined to the east. It forms a layer of 15 to 35 m of power, continuous over several kilometers along the north-south axis. A section of the deposit perpendicular to its extension shows, from east to west, the following succession (See profile opposite).
- Situated on the roof, black schists with dolomitic intercalations attributed to the summit of the Ordoviciens. - The talcous vein deriving from the transformation of overlying crystalline dolomites whose talc retains colour and structure. - In the axis of the deposit, lenticular inclusions (0 to 10m) of pure chlorites resulting from the transformation of pegmatite nuclei injected along the fault plane and massively transformed into chlorite. - Greyer talco-chlorite formations, resulting from the transformation of phyllates and micaschists alternating with residual sterile inclusions gradually passing to the sterile wall. - The wall corresponding to the gneiss and migmatites of the base of St Barthélemy.
Exploitation
The pit extends 1.5 km along a north-south axis at 1600 m altitude between a migmatitic gneiss wall to the west and a schistose and dolomitic roof to the east. Due to climatic conditions, the quarry is only worked 6 months of the year (from May to November) per season. The extraction of talc, in steps (ht 5 m) oriented along the axis of the deposit, is mechanized (excavators) and selective. Talc is shipped by cable car to the factory located in Luzenac, in the valley 1000 m below.
The treatment
The plant in
Luzenac processes, packages and ships all the ore extracted at Trimouns. The installations consist of:
- a sorting and selection unit including an optical sorting station for the valorisation of white ores,
- storage sheds for raw products,
- pendular grinding, fine grinding and selection, compressed air micronisation,
- two packaging units with pelleting presses, big bag loading device, two rotary paper bagging machines, installations for bulk loading in tanks,
- of finished goods stores,
- pilot station for industrial trials on new products and for optimization of existing processes.
Talc in the world: some figures
Annual world production is estimated at around 5 million tonnes excluding talc sold as ceramic raw material.
During the last three decades, the international market has been dominated by China, which is not only the world's leading producer with 2,300 kt/year but also the universal supplier with annual exports of around 1.3 million tonnes. Until 2008, this predominant role was linked to the practice of very low prices but also to the abundant production of exceptional qualities (very white talc). Since 2010, the trend has been reversed due to rising production costs, depletion of reserves and the emergence of a growing domestic market that is mobilizing all the country's resources.
- Second producer (1 million tons of talc per year), Europe has always played an important role thanks to the French and Finnish deposits. - In North America (USA and Canada), production amounts to 650 kt/year. - India is an important producer which is credited with 650 kt per year that it extracts mainly in the province of Rajasthan. Local production is almost totally absorbed by the domestic market. - Russia has limited reserves of medium quality in the Urals. Its annual production does not exceed 150 kt, it imports high-end products from China and Europe (around 15 kt/year) to satisfy the domestic market.
Jean-François Robert
(revised in June 2016)