
What is behind these words on the label of your make-up remover lotion? A huge quantity of small spheres (called micelles, hence, the micellar solution name), which target all the oily particles (make-up, sebum secretions …) that are on the surface of your skin. They will help you, by a quick and efficient move, to cleanse your face, your eyes and lips, or to remove make-up. A pharmacist, Valérie Schvartz gives explanations.
Micellar solutions are a good alternative to the cleansing cream/lotion team or to soap (that we always advise against for the face skin!). Depending on their formula, they are adapted to any kind of skin: normal, dry, combination and oily, even to the most sensitive.
How do they work?
Micelles are the smallest groups of surfactants that exist in a liquid. Some clues about surfactants.
It is likely you know them as emulsifiers, wetting agents, humectants, detergents (cleaners), foaming agents … as they are often named after what they do.
A molecule of surfactant has two ends (or heads):
• one, which is attracted by water (the so-called hydrophilic end)
• the other one is attracted by oil (so-called lipophilic, or hydrophobic end)
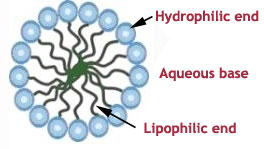 These opposing affinities lead to two physical effects: a lower interfacial tension between two liquid phases normally not miscible (hence, the possibility of a blend of water and oily substances) and when above a definite concentration, the surfactant molecules are attracted to each other to make spheres: the micelles.
These opposing affinities lead to two physical effects: a lower interfacial tension between two liquid phases normally not miscible (hence, the possibility of a blend of water and oily substances) and when above a definite concentration, the surfactant molecules are attracted to each other to make spheres: the micelles.
In micellar solutions, micelles are homogeneously hydro dispersed, the hydrophilic head of the surfactant being linked to water, while the lipophilic chain is inside the micelle. Some non …













