
ECHA (European Chemicals Agency) has just announced that its Risk Assessment Committee (RAC) has concluded on the harmonised classification of 15 substances. Among them, three (including Methyl Salicylate or Citric Acid) may be used in cosmetic products, and two are subject to a classification as CMR2 that eventually may lead to a ban on their use in cosmetic products.
The RAC, whose scientific opinions form the basis for regulatory decisions taken by the European Commission for chemicals, has adopted opinions on a harmonised classification of 15 substances. Four of them are listed in the European Glossary of Cosmetic Ingredients or in the CosIng database.
Methyl salicylate
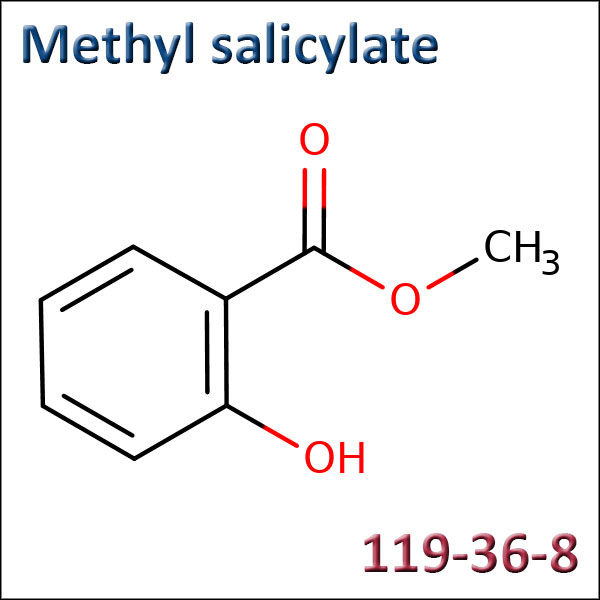 CAS: 119-36-8 - EC: 204-317-7
CAS: 119-36-8 - EC: 204-317-7
This substance is an industrial chemical mainly used in fragrances and cosmetics. The CosIng assigns it the functions of denaturing agent, perfuming agent and soothing agent.
The substance has no existing entry in Annex VI to the CLP Regulation.
RAC agreed to the proposal by France to classify the substance as harmful if swallowed (Acute Tox. 4) but assigned an acute toxicity estimate (ATE; oral) of 890 mg/kg bw (instead of 580 mg/kg bw). RAC further agreed to classify methyl salicylate as a substance that may cause an allergic skin reaction (Skin Sens. 1B) and as harmful to aquatic life with long lasting effects (Aquatic Chronic 3).
RAC did not agree to the proposal by France to classify methyl salicylate as a substance that may damage the unborn child (Repr. 1B; H360D) but classified the substance as suspected of damaging the unborn child …













