Lipopeptides are structures containing lipids chemically associated with proteins. They were first identified in 1866 by Hoppe Seyler, a German chemist and physiologist, but it was not until 1928 that the term lipoproteins appeared and their existence was no longer controversial.
In 1928, the French biochemist Macheboeuf (1900 - 1953) isolated a lipid-rich protein fraction from blood serum and showed that water-insoluble lipids can only be transported in plasma by their association with one or more specific proteins.
For his part, Jean Morelle, an independent French researcher, continued his work on skin biochemistry, focusing in particular on the skin's"buffer" function, i.e. its ability to restore its physiological pH after an imbalance such as when using alkaline soap. The role of amino acids was seen as important. He will therefore be interested in combinations of amino acids and lipids, such as lipopeptides, and will develop compounds that he will call Lipoamino acids. Since 1967, he worked on this type of compounds and filed numerous patents on this subject. He then demonstrated the interest of lipoamino acids as a biological factor in skin acidification.
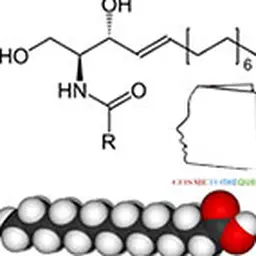
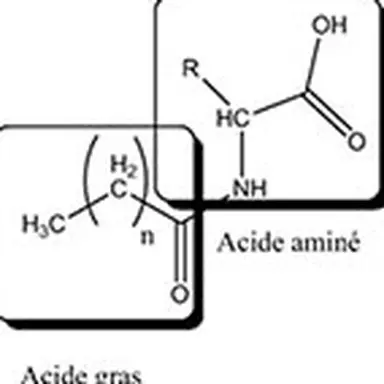
Lipoamino acids are a part of the lipopeptide family with one or more different amino acids present. There are 20 natural amino acids grouped according to the nature of their side chain: non-polar neutral (alanine, phenylalanine, leucine, isoleucine, proline, methionine, valine, tryptophan,…), polar basic (arginine, lysine, ornithine, histidine, citrulline), and polar acid (glutamic acid and aspartic acid). Amino acids can be either pure amino acids or amino acids from the total hydrolysis of proteins such as collagen, casein, elastin, keratin. The lipid part of lipoamino acids is composed of one or more fatty chains with various fatty acids in C4, C8, C11, C12, C16.
Due to the multifunctionality of amino acids and peptides, the lipophilic chain can be grafted according to different mechanisms (acylation, esterification, alkylation, amidation).
The simplest approach is based on the use of an acid chloride in an alkaline aqueous solution that contains the amino acids. This reaction was introduced by Schotten in 1844 and Baumann in 1886 and is still the most commercially used despite the formation of by-products (sodium alkanoate).
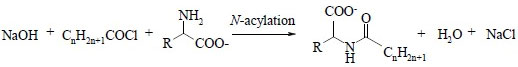 Schotten-Baumann N-acylation reaction of an amino acid (1)
Schotten-Baumann N-acylation reaction of an amino acid (1)
The esterification-based approach consists of reacting the protein hydrolysate obtained by chemical hydrolysis of proteins with fatty alcohols in an organic solvent such as toluene. The energy contribution is important since it is necessary to heat during 8h at 110°C.
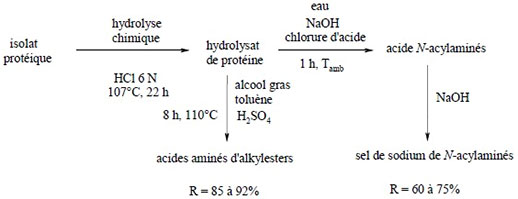 Obtaining amino acid surfactants from protein hydrolysate (1)
Obtaining amino acid surfactants from protein hydrolysate (1)
Lipoamino acids have a free carboxylic function, which allows them to be negatively charged or neutral depending on pH. The amine function is blocked. The coexistence of the hydrophilic characteristics of the carboxylic and hydrophobic function of the amino acid skeleton and the fatty chain makes lipoamino acids anionic surfactants. Another interest of lipopeptides is to have a structure close to the lipid-protein combinations present in large numbers in living matter.
Jean Morelle pointed out that the acidity of the skin coating depends on the carboxyl group content of amino acids whose amino functions are acylated by lipid chains. This acylation leads to the unmasking of the carboxylic acid function of the amino acid responsible for skin acidity on the one hand, and its capacity for self-protection against microorganisms on the other.
Jean Morelle had the lipoamino acids on which he worked produced by Rhône-Poulenc and then Givaudan Lavirotte. To date, Air Liquide and its subsidiary Seppic offer lipoamino acids in their product range.
1st generation lipoamino acids
Unneutralised lipoamino acids
When dispersed in water, lipoamino acids, if not neutralized, lead to a very acidic pH. For each amino acid bound, there is a free and active carboxyl function which leads to a large number of active carboxyl functions and an interesting biochemical attraction pole for structures comprising 22 to 24 different amino acids. The amine function is blocked.
| Nature of lipoamino acids | pH at 2% in city water |
| palmitoylglutamic | 3.5 |
| dipalmitoylcitrullinic | 5.6 |
| palmitoylcollagenic | 4.6 |
| undecylenoylcollagenic | 3.5 |
| undecylenoylcaseinic | 4.2 |
| caprylylcystinic | 3.8 |
| caprylyllcollagenic | 3.1 |
| caprylyceratinic | 3.2 |
| caprylylmethionine | 3.5 |
| Linoylcystinic (fatty acids from linseed oil) | 4.6 |
pH values for lipoamino acids in 2% city water (3)
Depending on the nature of the fatty chain combined with one or more amino acids, we also have a specific action: antiseborrheic, antifungal, antibacterial, stimulator of the cell development process, initiator role in protein synthesis, rapid passage through the skin, high affinity with keratin among others.
For example, undecylenic acid derivatives exhibit antimicrobial, anti-inflammatory and antifungal activity. The water-soluble lipoamino acids in C4 at most have an action on the cellular respiration which they amplify by increase of the cellular metabolism on the other hand they present a major disadvantage namely their very bad smell. The C8 compound is used for acne-prone skin. Dipalmitoylhydroxyproline (DPHP) has an action on skin elasticity. It hydrates and blurs the marks of time, firms tissues by stimulating the contraction of collagen fibres, protecting the dermal fibres from enzymatic lysis and presenting an antiradical action. It's a source of Hydroxyproline.
Several brands have used the benefits of lipoamino acids to launch cosmetic products: - Lenidermyl from Oberlin Laboratories (Upsa) with 10% palmitoylcollagen for soothing, anti-irritating activities. The marketing of the product has been stopped. - Chanel with the Crème Protection Nutrition Continue launched in the 1990s targeted the barrier function by normalizing it, action on keratogenesis using a palmitoylceratin. - Biotherm in several products such as the Sun Wrinkle Cream of the eponymous brand. - The company Seppic with lipacid DPPH offers a lipoamino acid whose content in Hydroxyproline and its power of biostimulation make it a central active of several products as well.
Neutralized lipoamino acids
Some lipoamino acids can be salified, such as caprylyl, undecylenoyl, lauroyl collagenic and keratinic acids. The salts obtained have surfactant properties with high foaming power. They behave like mild anionic detergents. According to the nature of the fatty chain they are endowed with specific capillary properties: anti seborrheic, antidandruff, revitalizing.
Dibasic lipoamino acids
The category of dibasic lipoamino acids is formed by the combination of a saturated or unsaturated fatty acid with basic amino acids such as lysine or arginine. Oleic, palmitic or stearic chains combined with lysine or arginine are the main fatty chains. These compounds protect fat from oxidation and skin from free radicals.
Compared to BHT, the oleic chain combined with lysine or arginine gives better protection than BHT used at a high dose.
The oleic chain linked to methionine has a strong antioxidant power. The nature of these lipoamino acids is perfectly compatible with our biological environment. This category of lipoamino acids is very little used in cosmetics despite its interest.
For more on this subject • Rondel Caroline, "Synthesis and properties of mixtures of novel polyfunctional lipopeptide surfactant molecules" Doctoral thesis from the University of Toulouse, February 2009 - Bijani Christian, "Lipoamino acids: absorption vectors for transmembrane administration of biomolecules" PhD thesis from the University of Bordeaux 1, March 2010 - Morelle Jean, "Lipoamino acids and cosmetology." Parfums cosmétiques savons de France, vol 3, n°2, février 1973 p. 82 -93 - Cotte Jean, "Milk, a material of the future for cosmetics", Milk (1991) 71,213-224, Elsevier/INRA - Martini Marie Claude," Introduction to Dermopharmacy and Cosmetology" 3rd edition, Lavoisier p. 76 to 77 - Morelle Jean, "Lipid peroxides, free radicals, aging and lipoamino acids, part 2" Parfums cosmétiques arômes n°80, 1988, p. 91 - 104
|
Contribution made by Régine Frick Régine Frick is a chemical formulation engineer and a CNAM graduate in innovation management, culture and marketing. She has worked for 12 years in research and development at Chanel parfum Beauté and has been a consultant for 6 years in her 3 favourite fields, namely communication, innovation and development for the cosmetic industries. From her technical and scientific skills, she uses as a consultant more particularly the science of mixtures associated with experimental designs to solve development problems. From her intellectual curiosity and her interest in society, she proposes to work with her clients on expected innovations. Her natural taste for communication has led her to write texts whose objective is to make scientific discourse accessible to everyone. |