Polymers based on the silicon atom in combination with oxygen, silicones find their origin in the work of Berzelius who isolated this element in 1824. Successive developments then led to the marketing of the first products in the 1940s by Dow Corning.
Silicones were quickly considered for multiple applications, particularly in cosmetics with first uses in hand creams for protective benefits(1). Their use then continued to grow, in parallel with the growing variety of products available. In the 1960s, volatile silicones significantly improved the sensory characteristics of antiperspirant roll-on products. Thanks to their touch and their specific behaviour, silicones have gradually conquered the solar, skincare and make-up segments since 1980. About ten years later, hair serums based on silicone gums appeared on the market and silicone emulsions were incorporated in 2-in-1 shampoos, making it possible to bring a hair conditioning effect to a shampoo cleansing formula. Silicone resins have made it possible to obtain dry films that are appreciated in long-lasting make-up formulations. In the 2000s, silicone elastomers brought a new sensory dimension and their optical properties have also been used more recently in primers and BB creams(2)(3). Today, these materials are used in virtually all cosmetic segments(3)(4) to meet a growing number of needs.
Synthesis and physico-chemical properties
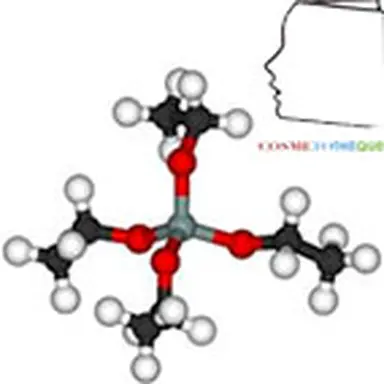
The term"silicone" includes a large number of structures whose common denominator is that they are polymers based on the siloxane bond, connecting a silicon atom to an oxygen atom. The best known in cosmetics are polydimethylsiloxanes (PDMS), characterized by the structure opposite.
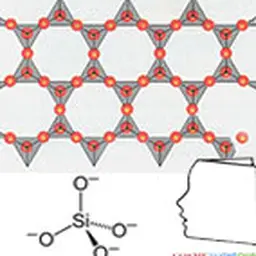
They are obtained by reduction of silica SiO₂ into silicon, from which dimethyldichlorosilane Me₂SiCl₂ is obtained under the action of methylene chloride. This intermediate is then hydrolyzed into dimethyldisilanol Me₂Si(OH)₂, which is not very stable and will polymerize by condensation to give a mixture of cyclic and linear polydimethylsiloxane oligomers. These low molecular weight products can then either be separated and used as is, or undergo additional chemical modifications, giving access to a wide variety of products.
Due to the differences in characteristics between siloxane and carbon-carbon bonds (longer length, more open angle, lower rotational energy), PDMS have very different properties compared to their hydrocarbon equivalents(5). The polysiloxane chain is highly flexible, which gives these polymers very low glass transition temperatures and allows them to remain in a liquid state at high degrees of polymerization. The polarity of the chain gives these products resistance to homolytic degradation, but its flexibility paradoxically makes them hydrophobic, with methyl groups orienting so as to form an apolar cloud around the skeleton. The result is a very low chain interaction and a particularly low surface tension which gives PDMS excellent spreading properties(6). Finally, their large free volume causes significant permeability to water vapour(7) but also to other gases such as oxygen.
Understanding these properties allows us to take stock of certain preconceived ideas sometimes heard about silicones, for example concerning their occlusivity level. Studies in-vitro and in-vivo on three traditionally used silicones confirmed their non-occlusive character: the results showed water vapour permeability similar to untreated skin and significantly different from vaseline, a recognized occlusive agent(7)(8)(9).
Silicones: a vast family
From the polydimethylsiloxane skeleton, it is possible to create new structures that sometimes have radically different characteristics and consequently bring distinct benefits to beauty products. Changing the degree of polymerization, creating a three-dimensional network or adding organic groups are the main levers used to generate the categories of silicones described below.
Table 1: INCI names of the most common silicones
| Types of silicones | Description | INCI Name |
| Poultry | Low molecular weight cyclic or linear siloxanes | Cyclomethicone Cyclopentasiloxane Cyclohexasiloxane Trisiloxane Dimethicone |
| Fluids | Linear siloxanes (with or without functionalities) | Dimethicone Dimethicone copolyol Aminodimethicone |
| Gums | High molecular weight siloxanes | Dimethicone Dimethiconol |
| Elastomers | Slightly crosslinked siloxane network | Dimethicone crosspolymer Vinyl dimethicone crosspolymer |
| Resins | Three-dimensional siloxane network | Trimethysiloxysilicate Polymethylsilsesquioxane Polypropylsilsesquioxane |
Polydimethylsiloxane fluids and gums
PDMS or"dimethicone" represent the oldest and most often encountered class of silicones in cosmetics. The sensory profile of these polymers, described as soft and non-greasy, has largely contributed to their success. Low molecular weight PDMS have a lower viscosity and their spreading properties help to promote the application of the formulations in which they are incorporated.
Their high molecular weight equivalents give cosmetic products a smoother feel and resilience (substantivity) properties. They are therefore often used in skin creams and lotions, but also in 2-in-1 shampoos or conditioners.
Emulsion polymerization techniques allow access to silicones with an even higher molecular weight(10), corresponding to theoretical internal phase viscosities above 100 million centistokes, opening the way to a very low sensory perception threshold and effects on the texture of the more marked formulas.
Volatile silicones
Silicones with a low degree of polymerization become volatile and are characterized by lighter touch and transient effects. Their volatility varies according to their structure (linear -Trisiloxane, Dimethicone- or cyclic -Cyclomethicone, Cyclopentasiloxane, Cyclohexasiloxane) and their degree of polymerization, thus allowing to modulate their effects. Their low latent heat of vaporization results in evaporation with no cool effect, unlike ethanol or water. They are among the most compatible silicones with organic raw materials and their very low surface tension(11) make them products appreciated when spreading plays a major role. All these characteristics explain the wide use of these products as vehicles in most cosmetic segments.
Connected silicones
The addition of lateral silicone chains to the main skeleton makes it possible to obtain products connected to the particular rheology, illustrated by the Weissenberg effect(12). This new type of silicone is therefore distinguished by a potential for innovative texture effects, but also a"satin" sensory profile, distinct from that of PDMS and can also provide an immediate perception of hydration on the skin.
Silicone elastomers
By crosslinking the linear chains of polydimethylsiloxanes with each other, covalent three-dimensional structures corresponding to silicone elastomers are obtained. Depending on the preparation process, these networks can be solid(13), semi-solid (elastomer whose network is swollen by a fluid) or in aqueous suspension(14).
When introduced into cosmetics, silicone elastomers(15) were a great success, mainly because of their particularly pleasant sensory profile, characterized as silky or powdery. Their fluid absorption properties give a mattifying effect (sebum) but also thicken fatty phases(16).
More recently, hybrid elastomers(17)(18) have emerged. The incorporation of organic patterns of different degrees of polarity within the structure makes it possible to modify their properties significantly. Thus, the addition of polyethoxylenated units generated an elastomer (PEG-12 Dimethicone/PPG-20 crosspolymer) capable of incorporating an aqueous phase inside the elastomeric network, thus giving access to new textures and modifying the"typical" sensory profile of silicone elastomers by offering a fresh effect to the application(18)(19).
Silicone resins
By continuing to increase the density of the network, we obtain silicone resins. These are prepared by hydrolysis of tri-functional silanes (T resins) or tetra-functional silanes (Q resins), as opposed to linear polydimethylsiloxanes obtained with di-functional silanes. As a result, Q(20) resins have a denser network compared to T(21) resins, resulting in higher glass transition temperatures.
Silicone resins help to form films on the skin, lips or hair that are resistant to water, sebum and mechanical friction, especially in combination with volatile silicones. They are therefore frequently used in the make-up field, for example in the formulation of lipsticks without transfer. They are also appreciated in other forms of products or applications for which sustainability benefits are sought. In hair applications, the ability to form a film can also be used to achieve easy styling and volume benefits(22).
Silicone polyethers
Silicone polyethers are obtained by incorporating polyethoxylated or polypropoxylenated units into the polydimethylsiloxane structure (e.g. PEG/PPG-18/18 Dimethicone, Lauryl PEG-10 Tris (Trimethylsiloxy)silylethyl dimethicone). As with other functional groups, this can be done either by copolymerization or by grafting, thus generating a wide variety of structures. These groups give the polymer a more polar character, allowing the extreme to obtain silicones dispersible in aqueous media. By modulating the quantity of incorporated groups, emulsifiers are obtained, allowing for example to disperse aqueous phases in hydrophobic silicones or oils. Compared to an oil-in-water emulsion, this type of system maximizes the sensory benefits of silicones, which are then found in the external phase, and forms films that typically have better water resistance (23). As these emulsifiers are generally in liquid form, it is possible to formulate at room temperature, in the absence of compounds with a high melting point in the formulation(11). Some of these polymers also make it possible to facilitate pigment dispersion or to formulate polyol-in-silicone anhydrous systems and multiple emulsions, thus being able to meet specific needs, for example in the case of formulations with active ingredients with limited stability.
There are also silicones modified with other types of polar groups such as glycerin derivatives (Cetyl Diglyceryl Tris(Trimethylsiloxy)silylethyl Dimethicone), which represent additional options for the formulator(24)(25).
Silicone waxes
The incorporation of alkyl units into a polydimethylsiloxane chain tends to increase melting point and compatibility with low polarity organic oils. It is thus possible to obtain silicones (Stearyl dimethicone, C30-45 Alkyl methicone) in the form of waxes, used as consistency agents in sticks or cream forms. These also present properties of substantivity and water resistance, benefits often sought in the field of sun products, in which their better compatibility with organic filters is an additional asset. Finally, the presence of these alkyl groups results in an occlusive character, providing moisturizing properties(26)(27).
Phenyl silicone
The presence of phenyl substituents gives these silicones(28) higher refractive indices (1.46 to 1.58 depending on the degree of substitution) and thus leads to brightness benefits in hair or make-up formulations. They are thus appreciated in glosses, but also lipsticks in which their increased compatibility with certain organic products and their aid in pigment dispersion are additional assets. In antiperspirant products, they mask the whitening effects that can be caused by certain salts used.
Amino silicones
The insertion of amine patterns into a polysiloxane aims to increase affinity with the hair. The presence of nitrogen atoms improves the deposition of these polymers on the hair fiber when the latter is damaged. This is explained by the electrostatic interaction between the positive charges carried by the amino groups which quaternize in aqueous medium and the overall negative charge then carried by the hair. In order to maximize this interaction and thus provide a more intense effect, quaternized silicones can be used. Since hair formulas are generally based on water, these products are often offered in the form of silicon-in-water emulsions to facilitate their incorporation. In this case, in addition to the molecular weight and amino group density of the polymer in the internal phase, the particle size and the emulsifying system used are key parameters that influence the deposition on the hair and therefore the effect obtained. This brings us back to another common misconception about silicones in this respect. This deposition is necessary in order to provide the expected conditioning benefits and depends on many parameters such as the type of silicone, the formulation, any cationic polymers used or the rinsing step. It has been shown that the level of deposition of an amino silicone(29) formulated as a shampoo does not increase with the number of uses and that it can be easily removed with a silicone-free clarifying shampoo(30).
Amino silicones have been used for many years in hair applications (2-in-1 shampoo, conditioner) to provide a"conditioner" effect, including detangling, softness, shine, volume and reducing electrostatic effects between hair fibers. The properties of the films formed by these silicones on the hair also make them relevant to meet other needs such as improving the durability of colour effects(31)(32), frizz control(33) or protection against damage caused by thermal treatments such as drying or smoothing(34) and mechanical treatments(35).
Acrylate silicones
Unlike most silicones, this product category (36) is built on an acrylic skeleton on which silicone groups are incorporated (Acrylates/Polytrimethylsiloxymethacrylate Copolymer). The acrylate function aims to improve affinity with the skin and to obtain film-forming agents with excellent friction resistance properties(37). Dendrimeric silicone groups allow the flexibility of the resulting films to be modulated and also increase sebum resistance, improving the potential for resistance to organic oils and meal resistance for lip products. Thanks to these characteristics, acrylate silicones are appreciated in make-up or suncare products, for which particularly high durability effects are sought.
Toxicological and environmental aspects
Due to their use for several decades in numerous applications, particularly in cosmetics and the medical field, silicones are among the most studied products from a toxicological and environmental point of view(3)(4)(38). Some PDMS and silicone elastomers are used, for example, for the siliconization of syringes, the treatment of scars or cardiac implants such as pacemakers.
Polydimethylsiloxanes are not biodegradable by nature (non-organic and therefore not digestible by micro-organisms), but they degrade according to different physico-chemical processes depending on the environmental compartment in which they are found. In the air (troposphere), the most volatile are attacked by OH free radicals under the influence of UV radiation. In water, they adsorb to suspended particulate matter that settles as sludge during wastewater treatment processes. These sewage sludge are intended either for energy recovery (incineration) or for land application in which a degradation of the polydimethylsiloxane chains catalysed by clays takes place. In all cases, the end degradation products are silica, carbon dioxide and water.
Conclusion
The success of silicones in cosmetics is explained by the specificity of their structure, based on the silicon-oxygen bond, and the resulting physico-chemical properties. The latter typically result in a differentiated behaviour in formulation compared to products derived from organic chemistry. The wide variety of silicones developed since their introduction in cosmetics over 60 years ago and the diversity of associated benefits has enabled them to be used in the vast majority of segments. Suppliers, for their part, continue to innovate in order to offer formulators new options to meet their product development imperatives and market trends.
Bibliography
1. 2. Soft Focus Anti-Aging Optical Effects. Dow's Corning. 3. 2016. form 27-1624-01. 2015. form 27-1550-01. 5. Why silicones behave funny. 6. Silicones: preparation and performance. 7. Silicones as Non-occlusive Topical Agents. 8. 9. Petrolatum: A useful classic. 10. Dow Corning(r) HMW 2220 Non-Ionic Emulsion. Dow's Corning. 11. Ghirardi, D., De Backer, G. Goodbye to Grease. Soap, Perfumes & Cosmetics. June 1993. 12. Dow Corning(r) 3901 Liquid Satin Blend. Dow's Corning. 13. 14. Dow Corning(r) 9509 Silicone Elastomer Suspension. 15. http://www.dowcorning.com. 16. Starch, Mr. 2002. Dow Corning paper. form 27-1060-01. 17. [Online] [Cited: August 09, 2016.] https://www.dowcorning.com/content/personal/silicone-organic-elastomer.aspx. 18. Dow Corning(r) EL-7040 HydroElastomer Blend. Dow's Corning. 19. Van Reeth, I., Bao, X.R., Dib, K., Haller, R. 11, November 2012, Cosmetics & Toiletries, Vol. 127, pp. 802-806. 20. 21. 22. 23. Van Reeth, I., More, M., Hickerson, R. 24. Dow Corning(r) ES-5600 Silicone Glycerol Emulsifier. Dow Corning. [Online] [Cited: August 09, 2016.] https://www.dowcorning.com/content/personal/personalskin/ES-5600.aspx. 25. 2014. form 27-1463A-01. 26. Wilson, A., Harashima, A. Yokohama: s.n., 1992. 27. 28. 29. 30. Zhu, J., Johnson, J., Van Reeth, I. The Beauty of Silicone in Hair Care Appplications. 2015. 27-1550-01. 31. Color Lock in Hair Care. 32. Dow Corning publication. 2000. Form 27-055-01. 33. Dow Corning(r) CB-3046 Fluid. Dow Corning. 34. ProtectTress. Dow Corning. 35. Silicones for Hair Strenghtening. 36. Dow Corning(r) FA 4001 CM, FA 4002 ID, FA 4003 DM & FA 4004 Silicone Acrylate ID. Dow Corning. 37. Van Reeth, I. Euro Cosmetics, pp. 18-24. 38. Silicones in the Environment. [Online] [Cited: August 09, 2016.] http://www.silicones.eu/sustainability-environment/silicones-in-the-environment.
| Contribution made by Jean Luc Garaud Jean-Luc GARAUD obtained his engineering degree from ESCOM (École Supérieure de Chimie Organique et Minérale) in 2001 and completed it with a Master in Industrial Cosmetology at IPIL (Institut de Pharmacie Industrielle de Lyon). He then joined Dow Corning in 2002 as a technical support engineer for cosmetic applications, then moved into activities focused on innovation and the development of new concepts, products and methods. He has co-filed some fifteen patents. |
| About Dow Corning Founded in 1943 specifically to explore and develop the potential of silicones, Dow Corning is a world leader in silicon-based technology and innovation. Dow Corning is a wholly owned subsidiary of The Dow Chemical Company. |







![By Rock Currier (http://www.mindat.org/photo-237295.html) [CC-BY-3.0 (http://creativecommons.org/licenses/by/3.0)], via Wikimedia Commons](https://d2aabgjce9enf.cloudfront.net/main/media/content/5/c/5cf74a4a92ef1b89e0549ec097048f7d3268ec4a--sm-noborder.webp)
![Par Ben Mills (Travail personnel) [Public domain], via Wikimedia Commons](https://d2aabgjce9enf.cloudfront.net/main/media/content/5/b/5b62847bc4229696df593a6b14899855ab56385e--sm-noborder.webp)
![Par Xvazquez (Travail personnel) [CC-BY-SA-3.0 (http://creativecommons.org/licenses/by-sa/3.0) ou GFDL (http://www.gnu.org/copyleft/fdl.html)], via Wikimedia Commons](https://d2aabgjce9enf.cloudfront.net/main/media/content/5/5/55b47c064570b73285696cf00e7203d6ab77a89b--sm-noborder.webp)