
PAO, e-mark, open book… You may think you know all there is to know about these regulatory logos that you print on your cosmetics labels almost systematically. But what if you don’t? And what if, in your ignorance, you are doing more harm than good? FEBEA Director of Scientific and Regulatory Affairs Anne Dux addressed the topic at a question-and-answer session on the cosmetics Regulation at the Beyond Beauty trade show.
The session was pleasant and practical, and has become a traditional feature of cosmetics events (the Chartres Congress, cosmetics trade shows, etc.): before a packed room, the regulatory experts from FEBEA, France’s Federation of Beauty Companies, discussed all aspects of the questions asked by industry professionals. Last 11 September, at the Beyond Beauty trade show, Anne Dux and Catherine Bramaud were the experts in question. Many of the questions they answered had to do with the use and conditions for use of logos on cosmetics packaging.
PAO / BBD
Is it possible to have both a PAO
and
a BBD?
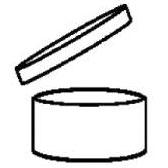 The Regulation explicitly stipulates that products must feature one
or
the other. Don’t forget that the date of minimum durability (incorrectly referred to as the BBD) must be indicated for products whose durability is less than 30 months. It is not required for products with greater minimum durability, but, for these products, the packaging must indicate the period after which the product is safe after opening: the PAO, represented by the ‘open jar’.
The Regulation explicitly stipulates that products must feature one
or
the other. Don’t forget that the date of minimum durability (incorrectly referred to as the BBD) must be indicated for products whose durability is less than 30 months. It is not required for products with greater minimum durability, but, for these products, the packaging must indicate the period after which the product is safe after opening: the PAO, represented by the ‘open jar’.
And yet, Ms Dux points out, providing both dates is not prohibited. The final decision is up to the responsible person. On the other hand, neither the …













