Gel varnishes first appeared in the United States in the 1980s, but met with limited success. At the time, lamp and gel manufacturers themselves had not succeeded in developing high-performance products. By the late 1980s, many companies had withdrawn their products from the market. But at the end of the 90s, gel varnishes came back on the scene, with very improved formulas, designed to work with a precise wavelength and light intensity.
In 2010, 63% of nail salons in the USA offered gel varnishes to their customers. The various innovations in gels that appeared in the 2000s, and 3D gels,"soak off gel" and"hybrid varnish gels" have allowed this category to break through.
Introduction
In nail make-up, hold and remanence have always been a major concern, both from the users' point of view and, by extension, from the manufacturers'. A nail polish works much like a decorative coating that must withstand wear more than any other make-up product. What makes things much more complicated is based on several factors, including the use of hands and the nature of nail polish.
- Concerning the use of hands, the problem is that almost everyone uses their hands and especially their fingers for a whole series of personal, household or professional tasks. As a result, these fingers are exposed to a multitude of mechanical and chemical stresses. - Regarding the product itself, the challenge with a nitrocellulose-based nail polish is that the coating is a combination of individual polymeric molecules, nitrocellulose and modified polymers, combined with plasticizers, pigments, effect pigments and other additives. The film actually has no internal structure, i.e. no cross-linking allows these polymers to hold together. Plasticisers are external to resins. Therefore, many causes can lead to film alterations that result in flaking and loss of gloss. - Another aspect is the adhesion of nitrocellulose and other polymers to the nail plate, which can be affected by a number of factors. Physical differences such as the flexibility of the nail, its water content, surface roughness play a role. Another key factor is the preparation of the nail before application: the nails should be as clean as possible, with no oily residue left.
Since there is little or no influence on the use of hands, improving the resistance of varnishes depends on how to optimize wear and therefore on its formulation. Attempts to prolong the varnish's resistance have consisted for an important part in reinforcing the polymeric mesh of the residual film, and especially its coherence, so that the product has a better resistance. In practice, this consists of the use of acrylate/methacrylate copolymers and terpolymers that contain polar groups. The main idea is that these substances will form by polymerization a more homogeneous film-forming assembly on the nail surface and thus develop a better interaction between the polymer and the nail plate.
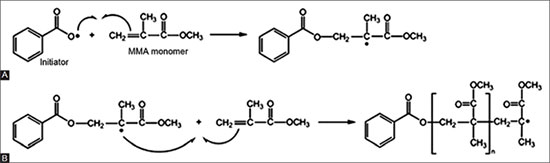
The practices initially led to systems in two parts, one product being in the form of a liquid, the other of a powder. These products are called"gels". They are used mainly to sculpt the nail or, almost exclusively, to be applied on false nails. The liquid contains acrylate and methacrylate monomers, the polymerization initiator powder and some additives. The principle is that the monomers, under the action of free radicals, will polymerize to form a resistant polymer. This initiator is generally sensitive to heat to generate free radicals and to be at the origin of the radical polymerization of liquid monomers. A good example is the polymerization of methyl methacrylate, initiated by benzoyl peroxide. Exposure to heat causes an O-O bond, generating two free radicals that initiate polymerization as shown in the diagram below.
Gel varnishes
However, these systems are very difficult to use because they are impractical. So we quickly switched to a new type of product, nail polish gels. These products are obtained by hybridization of traditional varnishes and acrylic gels. They are also called"Hybrid brush-on gel polishes". Gel coatings offer many advantages over these two-part systems. First, the photoinitiators are incorporated into the system rather than being added at the time of application. The thermosensitive initiators could not be used in this way because the product would have started the polymerization reaction in the bottle: others had to be found. It's the UV light. From this point of view, this technology is based on a principle widely used in surface coating techniques to improve wear resistance, or the glass bonding . So we're dealing with technology transfer. The other advantage is the greater control over the final polymer structure in terms of composition, degree of cross-linking and three-dimensional structure.
Hard gels and soft gels can be distinguished quite quickly. They can be applied on an extension of the nail (false nails) but especially on the nail itself. Hard gels require mechanical means to be removed, while soft gels, the famous"soak-off gels" can be removed more easily. In both cases, the films are not formed solely by evaporation of the solvent. The gel, in fact, is formed by exposure to UV radiation, which converts it from liquid to solid. UV light interacts with a photoinitiator to generate free radicals. For these"photoinitiators" to work, they must absorb enough energy (UV light) to create an excited state. Then the molecules dissociate to generate free radicals. And these interact with the acrylic monomers and oligomers in the gel to initiate polymerization.
Acrylic monomers and oligomers
Acrylates quickly proved to be the reference substances for these applications. In monomers such as ethyl methacrylate, acrylic acid and others, only one double bond is available for polymerization. As such, they can only form linear polymers.
To form two- or three-dimensional structures, functional molecules with two, three or more double bonds are required for polymerization. These are some examples of di-functional molecules: ethylene glycol dimethacrylate and polyethylene glycol acrylate homologues.
Different approaches can lead to a polymerized gel film with satisfactory hardness and flexibility. One is an optimized mixture of di- and tri-functional monomers and acrylates and methacrylates. In this case, the monomers determine the length of the chain, which increases flexibility, while multifunctional acrylate and methacrylate give strength and hardness. Methacrylate polymers tend to be more fragile than acrylate polymers. To increase the hardness of the film, a high crosslinking density must be achieved using tri-functional acrylates and methacrylates. Proper use of acrylates and methacrylates alone can also affect the properties of the final polymer.
The challenges in using monomers of this type relate to their odour and irritation potential. To avoid these problems, gel manufacturers mix monomers with oligomers. A special class is a polyurethane combined with an acrylate. These oligomers also give hardness to the film.
Based on commercial examples, film-forming ingredients are, for example, di-hema trimethylhexyl dicarbamate, hydroxyethyl methacrylate (HEMA) or hydroxypropyl methacrylate. Another example also contains di-hema trimethylhexyl dicarbamate and hydroxypropyl methacrylate, but also neopentyl glycol dimethacrylate and dimethacrylate isopropylidenediphenol PEG-2. In this case, since it is a high gloss topcoat, isopropylidenediphenol dimethacrylate PEG-2 increases the refractive index due to its phenyl functionality, leading to increased gloss.
Photoinitiators
As previously indicated, free radicals must be generated to initiate polymerization. This is the role of photoinitiators. To accomplish this function, they must absorb enough energy (UV light) to create an excited state.
The photophysics of many commercially available photoinitiators have been studied. Once the excited state is formed, the molecule can dissociate or reorganize hydrogen to generate a free radical. It is this that interacts with the monomers and oligomers to initiate polymerization. Examples of photoinitiators include hydroxycyclohexyl phenyl ketone, phenyldimethoxyacetophenone or trimethylbenzoyl diphenylphosphine oxide.
The molecular structure of the photoinitiator determines the wavelengths of the absorbed UV light, as well as the quantity. For example, hydroxycyclohexyl phenyl ketone has maximum absorption at about 240 nm and small absorption bands at 280 nm and 330 nm. Trimethylbenzoyl diphenylphosphine oxide has a maximum absorption at 295 nm and small absorption bands at 380 nm and 393 nm. It is preferable to use combinations of photoinitiators with different absorption spectra to ensure that the gel is completely processed throughout the film thickness. Especially when working with colours, as the addition of pigments reduces the depth of light penetration.
An important challenge to consider when working with photoinitiators is that their excited states are often modified by oxygen before they can generate radicals or the generated radicals react with oxygen. This explains why, after hardening, there may be a sticky layer on the nail surface, the polymerization being affected by oxygen on the surface, leaving a partially polymerized layer that should be removed. Photoinitiators excited at the film surface do not participate in polymerization.
The use of the right dose of photoinitiator is also critical for the development of gel systems. Too little leads to insufficient hardening, leaving a film too soft on the nails. Too much generates excess polymer chains that are too short or cross-linked, resulting in a film that is too hard and brittle and not flexible enough.
UV versus LED polymerization
UV rays with a different light source can be used. Conventional UV lamps use a compact fluorescent lamp (CFL) to produce light, when LEDs use a semiconductor diode. The big difference is the spectrum of UV light produced. The type of UV source can affect the curing of the film. CFL lamps have a wider distribution in UV, while LED lamps produce a narrower band. It is therefore important to use an appropriate light source. If the spectral output of the LED lamp does not match the absorption spectrum of the photoinitiator, polymerization will not occur, or only slightly. LFC lamps produce UV radiation over a wider range, they can induce polymerization of LED systems, but the opposite is uncertain. Furthermore, LED lamps do not generate as much heat as CFL lamps.
With regard to UV exposure, one concern was the amount of light used to cure the gel to the health of the user. Given the safety risks, one study concluded that"the risk of developing non-melanoma skin cancer is significantly lower than the risks of midday sun exposure"[JC Dowdy and RM Sayre, Photobiological safety evaluation of UV nail lamps, Photochemistry and Photobiology 89 961-967 (2013)].
A complete overview of the safety issues of these products in the professional world can be found in the available document by following this link .
Overview and outlook
Formulating a UV or hardenable LED gel varnish requires a detailed knowledge of monomers, photoinitiators and additives, such as pigments or special effect pigments. The final function will determine which combination types to choose. If you want to make a rigid gel or a soft gel, with or without extension, the combinations and levels of monomers, multifunctional acrylates and methacrylates, oligomers and photoinitiators are variable to obtain the desired result. Soft gels have been developed to avoid one of the disadvantages of hard gels, make-up removal. Hard gels require mechanical means, while soft gels are removed by dipping them in acetone. There are therefore many more soft gel systems on the market, hard gels often being reserved for professional use.
The trend is to develop gel systems that apply like traditional nail polishes. Many products are available on the market or for use in salons. And many brands now offer this type of speciality. Most of them are sold as systems including a base gel, a color coat and a top coat that must be cured. This also explains the popularity of gels from LEDs, which polymerize faster than UV gels.
A new interesting gel does not require an undercoat or top coat and is applied like a traditional nail polish but without solvent. This product is patent-pending . A further development combines a colour coat and a top coat that cures in natural light. Both contain an oligomer and the overcoat contains a photoinitiator, which, after application and drying, initiates the photopolymerization in contact with natural light.
Placing the varnish
Initially intended almost exclusively for false nails, nail application on natural nails is becoming widespread. However, the application of gel varnishes is almost exclusively done by a salon beautician. Why? Why? Quite simply because, even if it means keeping a varnish for two weeks, it might as well be applied very well. But above all, these gel formulas are a little thicker than a traditional varnish and therefore require a professional hand to dose the product.
This semi-permanent varnish is applied in the same way as a conventional varnish. The beautician files the nails, cleans them and then pushes back the cuticles. What is important when applying a long-lasting nail polish is the cleanliness of the nail: the nail polish must hang well. For this, the beautician uses a bonder before applying the base. This one allows a better adhesion of the products on the nail. The first hand made, we spend 30 seconds under a UV LED lamp… During this time, we make the second hand. The beautician then passes to the two coats of varnish, always with 30 seconds of drying between each coat. Finally, a top coat is applied, which also dries under the UV lamp.
The varnish application therefore takes between 25 and 30 minutes, with an express drying time. The evidence? At the end of the process, the beautician (heartily) cleans the nail with a cotton pad soaked in cleansing product. By reflex, we imagine the varnish damaged or streaked… wrongly! It's really dry!
With regard to removal, several solutions are available to users. The most recommended is to use a solvent adapted to this varnish, to leave 10 minutes on each nail. It is also possible to file the nail polish, but this must be done carefully so as not to damage the nail. And finally, it is frequent that after three weeks the varnish comes off on its own without damaging the nail.
Conclusion
The world of nail polish has evolved considerably in recent years. Even gel varnishes are not the only reason why Nail Art is so popular, even if they play a role that is regularly confirmed. Gel varnishes have come a long way since the 1980s when they began to be used. These products have undergone considerable evolution in terms of the types of references available and ease of use. With rapid advances in technology and chemistry, who knows what the future holds for gel coatings? One thing is certain, it will be very interesting.
Moreover, with market shares growing exponentially in recent years, nail polish products, which include nail polishes, have established their position, arousing the interest of the authorities. In France, the DGCCRF, in its latest inspection report of 249 establishments, published results that raise many questions: 43% of professionals are unqualified and 41% of the products analysed are non-compliant. ANSM and ANSES are also looking into the safety of these products: - the ANSM is in the process of drafting a recommendation aiming to ensure the safety conditions of the nail application for the consumers as well as for the professionals; - ANSES has contacted professional associations to analyse the various activities carried out by professionals, product exposure and the means of protection to be put in place. A report is expected in 2016.
References - FC Pagano, A review of nail polish: The industrial cosmetic, Cosm & Toil 126 5,372-380 (May 2011) • http://www.nailsmag.com/article/91808/the-science-of-gels • http://cosmetics.specialchem.com/tech-library/article/what-are-gel-nails - US Pat 5,772,988, Nail enamel compositions from acetoacetoxy methacrylate copolymer FC Pagano, AA Patil, RW Sandewicz, WL Anton and HJ Spinelli, assigned to Revlon Consumer Products Corp (May 10, 1996) • www.nailsmag.com/article/108201/hard-vs-soft-a-closer-look-at-gels - NJ Turro et al, Probing the reactivity of photoinitiators for free radical polymerization: Time-resolved infrared spectroscopic study of benzoyl radicals, J Am Chem Soc 124(50) 14952–14958 (2002) - Y Yagci, S Jockusch and NJ Turro, Photoinitiated polymerization: Advances, challenges and opportunities, Macromolecules 43(15) 6245-6260 (Jun 16, 2010) - NJ Turro et al, Photochemistry and photophysics of α-hydroxy ketones, Macromolecules 34(6) 1619–1626 (2001)
Example of a 3 in 1 product
ACTIVATOR INGREDIENTS ETHYL CYANOACRYLATE, POLYMETHYL METHACRYLATE. MAY CONTAIN BHA, 2-BROMO-2-NITROPROPANE-1, 36DIOL, NK ESTHER PEG-22, PEG-22 DIMETHACRYLATE, BENZO-18-CROWN-6. GEL-COLOR INGREDIENTS ETHYL ACETATE, ACRYLIC POLYMER, BUTYL ACETATE, ETHYL TOSYLAMIDE, BENZOATE ESTERS, N,N-DIETHYL-P-TOLUIDINE, STEARALKONIUM BENTONITE, NITROCELLULOSE, ISOPROPYL ALCOHOL. MAY CONTAIN : titanium dioxide (ci 77891), d&c red #6 barium lake (ci 15850), fd&c yellow #5 aluminum lake (ci 19140), red iron oxide (ci 77499), black iron oxide (ci 77499), d&cC RED #7 CLACIM LAKE (CI 15880), FERRIC FERROCYANIDE (CI 77510), BISMUTH OXYCHLORIDE (CI 77163), POLYETHYLENE TEREPHTHALATE, ALUMINUM POWDER (CI 77000), MICA.
Example of recent gel varnish composition
BUTYL ACETATE, ETHYL ACETATE, NITROCELLULOSE, ACETYL TRIBUTYL CITRATE, ISOPROPYL ALCOHOL, TOSYLAMIDE/EPOXY RESIN, STEARALKONIUM BENTONITE, TRIPHENYL PHOSPHATE, GLYCIDYL NEODECANOATE/PHTHALIC ANHYDRIDE/TMP CROSSPOLYMER, AQUA/ WATER/EAU, ADIPIC ACID/NEOPENTYL GLYCOL/TRIMELLITIC ANHYDRIDE COPOLYMER, CALCIUM ALUMINUM BOROSILICATE, CALCIUM SODIUM BOROSILICATE, SYNTHETIC FLUORPHLOGOPITE, ISOSORBIDE DICAPRYLATE/CAPRATE, SILICA, DIACETONE ALCOHOL, ETOCRYLENE, KAOLIN, HYDROXYETHYL ACRYLATE/IPDI/PPG-15 GLYCERYL ETHER COPOLYMER, ACRYLATES/DIMETHICONE COPOLYMER, CORALLINA OFFICINALIS EXTRACT, TOCOPHERYL ACETATE, PHOSPHORIC ACID, TRIMETHYLSILOXYSILICATE, DIMETHICONE, CETYL PEG/PPG-10/1 DIMETHICONE, STYRENE/ACRYLATES COPOLYMER, POLYVINYL BUTYRAL, CARTHAMUS TINCTORIUS (SAFFLOWER) SEED OIL, BUTYLENE GLYCOL, MACROCYSTIS PYRIFERA (KELP) EXTRACT, HYDROLYZED CONCHIOLIN PROTEIN, METHYLPARABEN, PROPYLPARABEN, TIN OXIDE,[May Contain/Peut Contain/+/-:mica, titanium dioxide (ci 77891), iron oxides (ci 77491, ci 77492, ci 77499), fd&c yellow no.: 77491, ci 77492, ci 77499), fd&c yellow no.: 77891. 5 aluminum lake (ci 19140), ferric ammonium ferrocyanide (ci 77510), d&c red no. 6 barium lake (ci 15850), aluminum powder (ci 77000), bismuth oxychloride (ci 77163), d&c red no.. 34 calcium lake (ci 15880), d&c red no. 7 calcium lake (ci 15850), d&c yellow no. 11 (ci 47000), manganese violet (ci 77742), ultramarines (ci 77007), d&c violet no. 2 (ci 60725)].
| Contribution made by Jean Claude Le Joliff A biologist by training, Jean Claude Le Joliff was a man of R&D for many years. Successively in charge of R&D, then of Research and Innovation in a large French group of cosmetics and luxury goods, and after an experience of setting up a research centre (CERIES), he turned to innovation management. He has also been an Associate Professor at the University of Versailles Saint Quentin (UVSQ) and remains in charge of courses in the framework of several specialized courses: ISIPCA, IPIL, ITECH, UBS, UCO, SFC, etc. He is the founder of inn2c, an R&D and innovation consulting company. Consultant for several international companies, he has actively participated in projects such as Filorga, Aïny, Fareva, and many others. He created the Cosmétothèque®, the industry's first conservatory of crafts and know-how. |