While their first start was a bit slow, the CMR Omnibus now looks like a moving train. Scheduled to arrive each year, in order to transcribe the recent harmonised classifications of substances into the Cosmetics Regulation, they are well on track. The second has just been published in the Official Journal of the European Union on 25 November 2019, with just a few adaptations compared to the text notified to the WTO last June.
Article updated on 12 March 2020, to take into account the two Corrigenda (December 2019, March 2020) made to the initial text
After the publication of the first Omnibus text on CMRs, Regulation 2019/831, in May 2019, this Regulation 2019/1966 deals with substances classified as CMRs by Regulation 2018/1480, which will be applicable from 1 May 2020.
Key points of Regulation 2019/1966
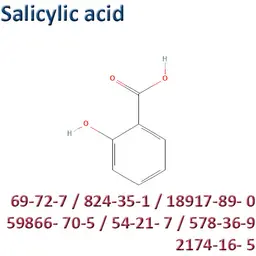
• An exemption from the prohibition is granted for salicylic acid and its salts, despite the classification in CMR 2, but its conditions of use are significantly restricted.
• As no other substances classified as CMR by Regulation 2018/1480 have been requested for use, they are all added to Annex II (Prohibited Substances) of Cosmetics Regulation 1223/2009 if they are not already included.
• 19 CMR substances that had been forgotten in the first Omnibus Regulation are also added to Annex II of the Cosmetics Regulation. • The text is applicable from 1 May 2020, with the exception of two corrections (for Oxyquinoline sulphate) which apply from 11 June 2019.
The “Whereas” of Regulation 2019/1966
Regulation (EC) No.1272/2008 of the European Parliament and of the Council provides for a harmonised classification of substances as carcinogenic, mutagenic or …