The first European Directive on cosmetics was adopted in 1976. 40 years later, the initial text has changed a lot, annexes have been added to it, the Directive has become a Regulation… but the objective has remained the same: guaranteeing consumer safety and the free circulation of products in Europe thanks to harmonized regulations to be applied exactly the same way in all its Member States. We attended the Cosmetics Europe Week in Brussels last June 13-17: here is a review of the major developments.
1976 – Adoption of the Cosmetics Directive 76/768
The Member States of the European Economic Community (now called the European Union – UE) decided to harmonize their national cosmetic regulations in order to ensure a high level of consumer protection and enable the free circulation of cosmetic products within the Community.
1979 – Establishment of the SCC (now SCCS)
The EU created a panel of independent experts (called Scientific Committee on Cosmetology), chosen for their scientific excellence and independence, to advise on safety of cosmetic ingredients in a transparent manner and on science-based reasoning.
Renewed several times over the years, this committee, now called Scientific Committee on Consumer safety, is internationally recognized and its opinions serve as the basis for regulatory decisions beyond the EU.
1980's – Positive list of preservatives
The EU – with the support of its scientific committee – set up a list of substances that can be used as preservatives in cosmetics and the conditions / concentrations that need to be respected.
Cosmetic companies cans only use those substances as preservatives. Any new preservative must first be evaluated, found safe by the SCCS and added to the list before it can be used.
1990's – Positive list of UV filters
The EU – with the support of its scientific committee – set up a list of substances that can be used as UV filters in cosmetics. Whilst the data requirements for new UV filters are high, they are clearly described by the SCCS guidelines and industry can operate in a predictable and transparent approval environment. This has allowed the EU industry to become the global leader in UV filters sun protection.
1993 – 6th amendment to the Cosmetics Directive
This amendment refined and completed the main principles of the EU Cosmetics Regulation which have since become the model and inspiration for many emerging regions of the world:
• Risk-based regulation aiming at a high level of consumer protection
• Mandatory safety assessment of every product placed on the market, to be carried out by a suitable and trained safety assessor
• Every product must have a comprehensive technical information file that is accessible to control authorities during in-market control
• Industry responsibility represented by the 'Responsible Person'
• Member States authority obligation to carry out an effective in-market control
• Full ingredient labelling using INCI nomenclature; Consumers can, with help of the INCI (International Nomenclature of Cosmetic Ingredients) name, recognise ingredients on the label when buying a cosmetic product anywhere in the EU.
1997 – Addressing new challenges for risk assessment and risk management
In the wake of the BSE food crisis, the EU Commission decided to clearly separate the functions of risk assessment (SCCS) and risk management (legislation) to ensure their independence. Since then, the two responsibilities are managed by different Departments /Directorate Generals in the EU Commission.
2000 – First alternative method validated /accepted for cosmetics
The term 'alternatives' was coined by the distinguished physiologist David Smyth in his 1978 book
Alternatives to Animal Experiments
. It is used to describe any change to established scientific procedures that will result in the replacement of animals.
Alternative skin irritation tests became available in 2000 after they had been validated by ECVAM and adopted by the EU and OECD.
2001 – A new approach to chemical legislation in the EU with REACH
Chemicals placed on the EU market –be it as such or as part of a finished product, need to be registered with a safety data package and a human and environmental safety assessment. Cosmetic ingredients are covered by this registration obligation.
REACH is the most important piece of environmental legislation for the substances used in cosmetics.
2003 – 7th amendment to the Cosmetics Directive
Besides refined consumer information requirements on the presence of certain allergens and on product durability, this amendment brought two major changes:
• The animal testing bans for cosmetic ingredients, which up to this time were dependant on the availability of alternative methods, were linked to fixed deadlines (2009 and 2013) – irrespective of the scientific progress and availability of alternative methods
• A categorical, hazard based ban in cosmetics of the use of substances classified under the Chemical s legislation as CMR Cat. 1A or 1B (irrespective of whether such use in cosmetics is safe or not
2004 – Enlargement / TAIEX
During several rounds of EU Enlargement a total of 22 new Member States adopted the EU Cosmetics Directive since 1976 and implemented it into their national laws.
Each time, significant preparatory work was carried out between the accession countries, both at industry level and authorities' level, and the EU Commission and industry associations. So-called TAIEX – Technical Assistance and Information Exchange instrument - seminars allowed the accession countries to get a good understanding of the harmonised EU legislation, including on cosmetics. This ensured a smooth transition and integration for these countries and their products into the EU internal market.
2009/2013 – New EU Cosmetics Regulation enters into force
Followed by an adoption in 2009, the year of 2013 finally marks the 'apotheosis' of the harmonised cosmetics legislation in the EU. Changing the legal form from a Directive to a Regulation means that the EU legal text no longer needs to be transposed into the national legislation of the Member States (sometimes slow and imperfect), but the EU text is as such the directly applicable law in all EU Member States.
The new Regulation also introduced a single, electronic product notification system (CPNP) that replaced the more than 20 different national systems existing at that time.
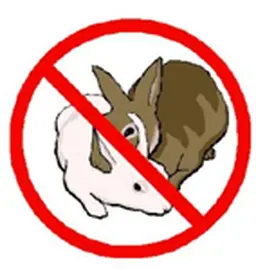
2013 – Animal testing ban of cosmetics ingredients came fully into effect
The testing ban was already in place since 2009 (but testing for certain complex endpoints could still be done outside the EU). Since 2013 it also became prohibited to market products that contained ingredients which were tested on animals (including for the toxicological endpoints that were exempted in 2009). As from 2013 the ban is complete both for testing and marketing.
2016 – Cosmetics regulation turns 40
And it keeps changing… thanks to CosmeticOBS, you will not have to wait another 40 years for regulatory news: you will get updated on any modification on a daily basis!
Source
•
Cosmetics Europe