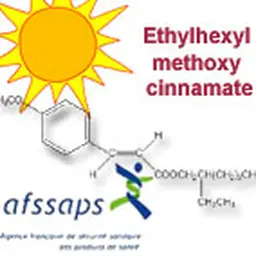
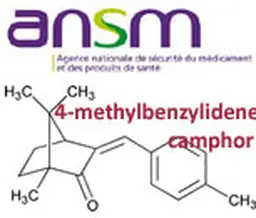
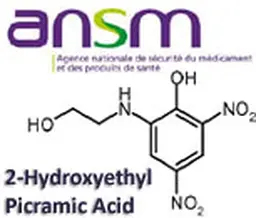
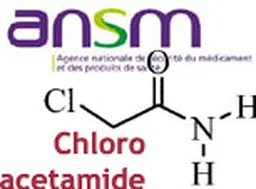
As it does every summer, even more since the Agency has been reshaped and the transparency requirements enhanced, the French ANSM has released its activities report for last year. A view of the cosmetic products sector, with figures about clinical tests, cosmetovigilance, inspections …
* ANSM: Agence Nationale de Sécurité des Médicaments et Produits de Santé - National Agency for the Safety of Medicines and of Health Products
Everyone may guess, in view of recent events, that this report deals by far with the Agency’s activities in the medicines sector. Cosmetics, nevertheless, are not put aside, and some pages concern them.
The first ones deal with the “cosmetic“ context of the ANSM: interface committee with industry’s representatives, position, in the Agency’s organization chart, of the Direction of therapeutic medical devices and cosmetics (DTMCOS), managed by Brigitte Heuls, a reminder of the regulatory frame (for 2012, the Directive, replaced on 11 July 2013 by the Regulation 1223/2009), the collaboration with the Directorate-General for Competition, Consumer Affairs and Prevention of Fraud (DGCCRF) to control the market …
Then, the figures are given for the different sectors of activity.
Clinical tests
The ANSM allows biomedical research on cosmetic products. Its work is to check whether the product’s and consumer’s safety is guaranteed.
In 2012, the ANSM was asked only seven times for an authorization (instead of 17 in 2008, 12 in 2009, 13 on 2010, 14 in 2011). The report states that three of these requests have …