On 8 July 2015, the ANSM published its Activity Report for the year 2014. After medicines, biological products, medical devices… The Observatory has searched in the"Other products" section for the few passages that concern cosmetics.
162 pages in all, and just a few devoted to cosmetic products: since its overhaul, the National Agency for the Safety of Medicines and Health Products has not devoted most of its activities to this area. See page 85 for the first mention of cosmetics in this report.
Cosmetovigilance
A reminder first: since 11 July 2013, the date of entry into force of European Regulation 1223/2009/EC on cosmetic products, which requires the reporting and transmission of serious adverse reactions (SUE), the ANSM also acts as a platform between the competent European authorities, industry and end users for these effects.
In 2014, the Agency processed 193 reports of cosmetovigilance (compared to 157 in 2013). Of these, 86 reports are serious.
Market control
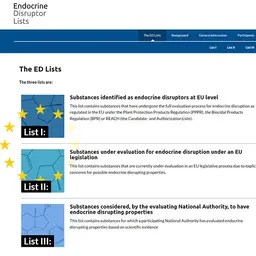
The ANSM also carries out missions to evaluate the toxicological profile of substances used in the composition of cosmetic products. Most often, this evaluation work gives rise to active cooperation with other institutions, in particular with the DGCCRF and the ANSES. Several families of substances are the subject of in-depth expertise, in particular lead, vitamin A and endocrine disrupting substances.
Highlights for 2014 : - the decision to suspend the placing on the market free of charge or not, …