Even if the word is not used in the document, cosmetovigilance is provided for in the Cosmetics Regulation (which replaces the old Directive since 11 July 2013). To sum it up, it is mandatory to notify to the authorities the serious undesirable effects (SUE), linked to the use of cosmetics.
A new requirement in the Regulation
The Cosmetics Directive did not ask for a harmonized European system of cosmetovigilance.
On the other hand, it required, in its Article 7 bis that "The manufacturer (…) shall for control purposes keep the following information readily accessible to the competent authorities of the Member State concerned at the address specified on the label (…):existing data on undesirable effects on human health resulting from use of the cosmetic product." In other words, the data shall be available in the product file in case of inspection (in France, by the Afssaps or the DGCCRF), but it is not mandatory to notify them to the competent authorities.
Further, this document required that "Member States shall ensure that the information (…) shall be made easily accessible to the public by any appropriate means, including electronic means." Consumers are allowed, since 2006, to ask directly the responsible person of the placing on the market, in the manufacturing companies, about the reported undesirable effects.
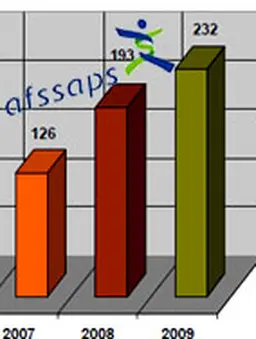
France at the forefront
In 2004, the French Code for Public Health, through the LOSP law on Public Health ("Loi d’Orientation de Santé Publique", article 139), enforced a national cosmetovigilance. It is a surveillance system …