They did not exist until now and derive from one of the novelties of the Chinese cosmetic regulation: from January 2021, any product import will have to be accompanied by a GMP certificate issued by the control authority of its country of origin. In France, the ANSM will be in charge of issuing them. During the Perfumes & Cosmetics Congress of the Cosmetic Valley, which was held digitally on 4 and 5 November 2020, Thomas Lecardez, Head of the INSMAR division of the ANSM, detailed the framework and procedures of this new activity of the Agency.
This GMP certificate will be one of the passports for the export of cosmetic products to China (and possibly to other countries outside Europe). To prepare for the January 1st deadline set by the new CSAR (Chinese Cosmetic Regulations), ANSM has been working with the various professional organisations to develop the most efficient system and procedures possible.
And the Decree n°2020-1337 of 2 November 2020, published in the Official Journal of French Republic on 4 November officially allowed the Agency to issue “a certificate attesting [that any exporting establishment] complies with the good manufacturing practices mentioned in Article 8 of Regulation (EC) No.1223/2009 relating to cosmetic products”.
“This is the first step,” commented Thomas Lecardez. “The second will be the publication by the Agency of a Decision of its Director General which will list the different procedures leading to the production of a GMP certificate. We are talking about ten or so documents that will be required from the various operators to obtain their certificate”.
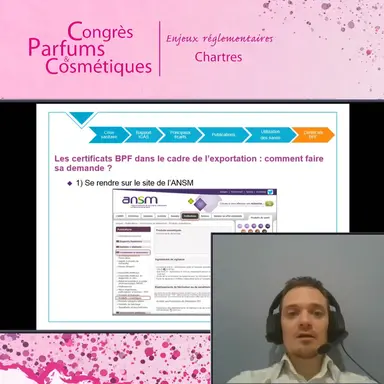
An online request
The certificate application file can be submitted online, on the demarches-simplifiees.fr website (where declarations of establishment are already made and with the same account) or on the ANSM website, under …













