Often banned by the consumerist press, they are considered dangerous ingredients although essential to the preservation of cosmetics. During a workshop organised for journalists on 11 December 2018, led by Françoise Audebert, Regulatory and Scientific Advisor and Anne Dux, Director of Regulatory and Scientific Affairs, FEBEA decided to take up the challenge in order to restore the truth about these often criticised ingredients.
As its name suggests, a preservative is used to preserve the product and prevents microbial contamination. Thanks to it, the consumer is guaranteed to use his care without risk to his health and for a relatively long time.
Preservatives in cosmetics: why?
Like humans, bacteria need water to grow.
In a cosmetic, bacterial proliferation is possible from 20% water in the formula.
“The preservation of a cosmetic product will therefore depend on its formula and water concentration,” explained Anne Dux.
Just like antibiotics, preservatives are not always automatic! Some products do not require their use:
• Products formulated without water such as powders, lipsticks or oily serums • Cosmetics containing ingredients that make microbial proliferation impossible, such as alcohol
• Care in the form of aerosols that contain a propellant gas (e.g. butane or propane)
To do without preservatives, it is also possible to formulate sterile cosmetics, using the UHT process. The only downside is that not all substances can tolerate this treatment.
Choosing the right preservative
In Europe, the ingredients used for preservation must be listed in Annex V of the Cosmetics Regulation. This positive list, established on the basis of the safety of substances evaluated by a committee of European experts (the SCCS), contains 59 entries.
In practice, only about twenty are used.
“Industrialists cannot choose just any preservative. Indeed, it must correspond to the germ that may develop in the product and this depends on the formula, pH or application area. For example, PHMB (Polyaminopropyl biguanide) is a preservative that does not sting the eyes, which is why it is used when formulating a product for this area,” said Anne Dux.
What about safety?
Preservatives are substances that kill germs, bacteria and fungi, so they are killers and therefore also potentially toxic.
“Their safety is systematically assessed. To qualify the hazard of a substance, it is necessary to have experimental data on pH, local toxicity, irritant potential, systemic hazards, etc… and then to assess consumer exposure. The authorized doses set correspond to a concentration 100 times lower than the dose that does not cause a toxic effect (NOAEL),” explained Françoise Audebert.
Why are they being questioned?
The questions we mostly ask about the preservatives are about their allergenic and irritating potential. And it should be noted that the more a person is exposed to an allergenic substance, the more likely they are to trigger an allergy.
“This is exactly what happened with methylisothiazolinone (MI). This case deserves all the lessons we can learn from it,” Anne Dux insisted. “MI is a preservative used since the 1960s, first in combination with methylchloroisothiazolinone (MCI), particularly in rinse-off products, shower gels or shampoos. But these two preservatives caused allergies. The authorities have therefore asked manufacturers to limit their use. This is why beauty professionals have turned more to parabens, which also have a wide spectrum of activity against microorganisms. But after the 2005 media crisis, parabens were abandoned, and the MI was again widely used.”
The problemis that MI is highly allergenic and has been used far too much, in too many products, including leave-on products, that significantly increase consumer exposure. As a result, the industry has faced a sharp increase in allergy cases.
“Morality,” according to Anne Dux, “you must not ban a conservative without a valid reason. It is important to vary the preservatives in the formulas.”
Parabens and Phenoxyethanol, scapegoats for consumers?
According to Françoise Audebert and Anne Dux, these two preservative, hated by the general public, are not as harmful as we might think.
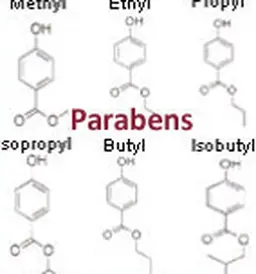
Parabens
Identity card
It is a family of synthetic substances derived from parahydroxybenzoic acid.
Their wide spectrum of activity makes them widely used preservatives (more than 50 years of use) and in several fields (pharmacy, food, etc.).
In cosmetics, the concentration of parabens is regulated.
In addition, they have been the subject of numerous evaluations by the SCCS in 2005, 2006, 2008, 2010, 2010, 2011 and 2013.
The controversy
“In 2004, Professor Philippa Darbre conducted a study that seemed to indicate a relationship between parabens and breast cancer. These potential links have still not been confirmed, but the controversy has indeed been launched. Different kinds of parabens are mainly “suspected of…”. To date, no study has confirmed these concerns, but the mention “paraben-free” is widely used on the packaging of our cosmetic products. The controversy is therefore constantly fuelled,“ explained Françoise Audebert.
Since 2015, only four parabens remain authorized (methyl-, ethyl-, ethyl-, propyl-, butyl-paraben) at a limited concentration. Their use is prohibited in leave-on products intended for application to the seat area of children under three years of age, as the risk of skin penetration is higher. For more information on the controversy over parabens, see the article Parabens.
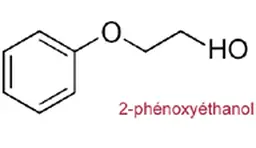
Phenoxyethanol
Identity card
“It is a glycol ester that is naturally present in green tea and chicory,” said Françoise Audebert.
It has a wide spectrum of activity and has been used for 40 years, in many fields but mainly in cosmetics.
Its maximum permitted concentration is 1%.
The controversy
In autumn 2008, the C2DS (Committee for Sustainable Development in Health) drew the attention of the ANSM to this compound present in cosmetics distributed in maternity wards. Following this request, the Agency investigated and published its recommendations in 2012. She recommends not using phenoxyethanol in cosmetic products for baby seats. “However, to conduct its studies, the ANSM used the NOAEL of a study that did not comply with OECD rules and that had many biases. These results have been brought to the attention of the European Commission and forwarded to the SCCS for re-evaluation. In 2016, the European Committee stated that the NOEL used by the ANSM was not good and estimated that phenoxyethanol used at 1% as a preservative in cosmetic products is safe for health, regardless of age group,” Françoise Audebert reacted. However, in 2017, the ANSM re-evaluated the safety of this preservative and maintained its position.
Anne Dux pointed out that “since the beginning of the controversy, the FEBEA has been urging its members not to comply with the recommendation of the ANSM, because we consider it to be scientifically unfounded.”
Very often criticized, however, preservatives are essential in products’ formulation.
Today, industry no longer has many authorized substances at its disposal. Cosmetics Europe’s Action Plan at the European level was therefore launched at the initiative of cosmetics professionals’ associations.
The goal, to defend preservatives, and above all to keep a wide range of substances, to ensure the safety of products… and consumers! Against all odds!