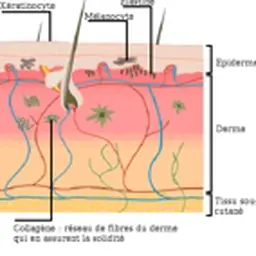
An acronym for substances that are Carcinogenic, Mutagenic (inducing permanent changes in the number and/or structure of the body’s genetic material) or toxic to Reproduction (e.g. by causing malformations of the sexual organs or impairing sperm quality).
CMRs are listed by the European Union in Regulation 1272/2008 on classification, labelling and packaging of substances and mixtures (known as the CLP Regulation) and classified in different categories (CMR1, CMR1A, CMR1B, CMR2), according to their degree of danger or the knowledge we have acquired about them.
Carcinogens
Carcinogen” means a chemical or mixture of chemicals that induces or increases the incidence of cancer.
Substances that have induced benign and malignant tumours in animals in properly conducted experimental studies are also presumed to be or may be carcinogenic, unless it is clear that the mechanism of tumour formation is not relevant to humans.
Proven or suspected human carcinogens.
The classification of a substance as a category 1 carcinogen is based on epidemiological data and/or data from animal studies.
A substance classified in Category 1 may be further distinguished:
Categoy 1A
Includes substances that are known to be carcinogenic to humans.
The classification in this category is largely based on human data.
Categoy 1B
Includes substances with suspected human carcinogenic potential.
Classification in this category is largely based on animal data.
Categoy 2
Substances suspected of being carcinogenic to humans.
The classification of a substance in category 2 is based on …