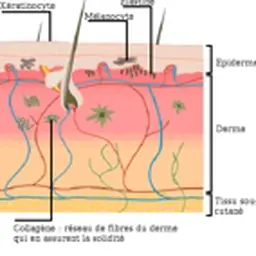
French Health Products Safety Agency.
Replaced on May 1st, 2012 by the ANSM, the Afssaps had been established in March 1999 to guarantee the efficiency, the quality and the proper use of all the products designed for human health: medicines and raw materials, medical devices, human biological products (blood products, organs, tissues, cells, gene therapy products and cell therapy), and, of course,
cosmetic products
…
It also monitored unexpected or
undesirable effects
associated with their use, via the
Cosmetovigilance
system.
It had to check laboratory testings, carry out inspections of manufacturing and research sites, send warnings, information or recommandations to the consumers or the professionals, issue reports on the evaluation of the safety of products or of cosmetic ingredients.
Also see
• The
ANSM Sheet